We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Léa Wick/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
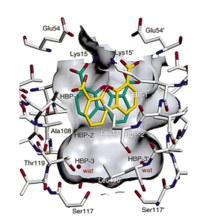
The dimer interface of the TTR is divided in two part, the inner and the outer binding cavity. The channel forming by dimerization has three symmetric binding pockets on each dimer parts. These pockets are called Halogen-binding pocket (HBPs) due to their ability to bind the iodines of thyroxine [3]<ref name="Labaudinière">Labaudinière R. Chapter 9 Discovery and Development of Tafamidis for the Treatment of TTR Familial Amyloid Polyneuropathy. Orphan Drugs and Rare Diseases. Aug 2014 202-229. DOI:https://doi.org/10.1039/9781782624202-00202</ref>. In the outer cavity are positioned the HBP1 and HBP1’ pockets, in the inner cavity are placed the HBP3 and HBP3’ pockets, and the HBP2 and HBP2’ pockets are at the interface between the inner and outer cavity | The dimer interface of the TTR is divided in two part, the inner and the outer binding cavity. The channel forming by dimerization has three symmetric binding pockets on each dimer parts. These pockets are called Halogen-binding pocket (HBPs) due to their ability to bind the iodines of thyroxine [3]<ref name="Labaudinière">Labaudinière R. Chapter 9 Discovery and Development of Tafamidis for the Treatment of TTR Familial Amyloid Polyneuropathy. Orphan Drugs and Rare Diseases. Aug 2014 202-229. DOI:https://doi.org/10.1039/9781782624202-00202</ref>. In the outer cavity are positioned the HBP1 and HBP1’ pockets, in the inner cavity are placed the HBP3 and HBP3’ pockets, and the HBP2 and HBP2’ pockets are at the interface between the inner and outer cavity | ||
| - | DDBF is bounded according two symmetric equivalent modes [2]<ref name=" | + | DDBF is bounded according two symmetric equivalent modes [2]<ref name="Petrassi">PMID:15869287</ref>. Indeed, DDBF wears a tricyclic ring system, with 2 hydrogen bond donors and 5 hydrogen bond acceptors, allowing to bound the dimer-dimer interface of the TTR cavity. [Pubchem,1]<ref name="National Center for Biotechnology Information">[https://pubchem.ncbi.nlm.nih.gov/compound/Dibenzofuran-4_6-dicarboxylic-acid Link text], PubChem Database. CID:3022(accessed on Dec. 26, 2019).</ref><ref name="Klabunde">PMID:10742177</ref>. Thanks to the complementarity of shape and hydrophobicity, DDBF enters nicely the outer portion of HBPs pockets [2]<ref name="Petrassi "/>. Besides, the tricyclic ring system interacts with Lys15, Val17 and Ala108 from two adjacent TTR subunits [2]<ref name="Petrassi"/>. Additionally, carboxylates at the position 4 and 6 of DDBF make electrostatic interactions at the entrance of HBP1 and HBP1’ with Lys15 on the ε-NH3+ groups[2]<ref name="Petrassi"/>. |
| Line 17: | Line 17: | ||
===Improvements=== | ===Improvements=== | ||
| - | To exploit the TTR inner cavity, DDBR can be ameliorate [1]<ref name="Klabunde">PMID:10742177</ref>. An additional substituent like an aryl ring could be link at DDBR thought a heteroatom or directly via a covalent bond [2]<ref name=" | + | To exploit the TTR inner cavity, DDBR can be ameliorate [1]<ref name="Klabunde">PMID:10742177</ref>. An additional substituent like an aryl ring could be link at DDBR thought a heteroatom or directly via a covalent bond [2]<ref name="Petrassi"/>. |
For example, a N-phenyl phenoxazine-4,6-dicarboxylate, called Phenox (1dvy), allows to create additional bonds, which increase the kinetic stabilization of TTR. Besides, Van der Waals interactions are established with Thr106, Lys15, Leu17 from to adjacent TTR subunits. Moreover, the carboxylate groups link not only Lys15 but also Glu54 (carboxylate groups must to be protonated at physiological pH). In the side chain Leu17, Leu110 and Thr119 hydrophobic interactions take place with the trifluoromethyl group [1] | For example, a N-phenyl phenoxazine-4,6-dicarboxylate, called Phenox (1dvy), allows to create additional bonds, which increase the kinetic stabilization of TTR. Besides, Van der Waals interactions are established with Thr106, Lys15, Leu17 from to adjacent TTR subunits. Moreover, the carboxylate groups link not only Lys15 but also Glu54 (carboxylate groups must to be protonated at physiological pH). In the side chain Leu17, Leu110 and Thr119 hydrophobic interactions take place with the trifluoromethyl group [1] | ||
<ref name="Klabunde">PMID:10742177</ref>. | <ref name="Klabunde">PMID:10742177</ref>. | ||
Revision as of 17:06, 6 January 2020
| |||||||||||