Sandbox Reserved 1102
From Proteopedia
| Line 1: | Line 1: | ||
{{Sandbox_ESBS_2019}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_ESBS_2019}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
== 5y6n- Zika virus helicase in complex with ADP == | == 5y6n- Zika virus helicase in complex with ADP == | ||
| - | 5y6n is a 1 chain protein structure. It’s the only helicase belongs to [https://en.wikipedia.org/wiki/Zika_virus zika] virus. | + | |
| + | 5y6n is a 1 chain protein structure. It’s the only helicase belongs to [https://en.wikipedia.org/wiki/Zika_virus zika] virus. | ||
| + | |||
| + | |||
== Function == | == Function == | ||
Revision as of 15:24, 12 January 2020
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
Contents |
5y6n- Zika virus helicase in complex with ADP
5y6n is a 1 chain protein structure. It’s the only helicase belongs to zika virus.
Function
Zika is an double-stranded RNA virus. The genome replication of double-Stranded RNA virus need intervention of helicase.
Protein from Zika virus of a protease domain at its N terminus and a helicase domain at its C terminus (8–10). The multifunctional NS3 helicase, belonging to the superfamily 2 (SF2) helicase family, is an essential enzyme involved in the cycles of ATP hydrolysis and behaves as a motor protein in RNA unwinding (11, 12).
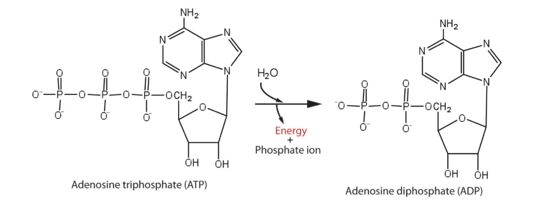
helicase domain that has 5'-triphosphatase activity of ZIKV performs the critical and indispensable function of unwinding double-stranded RNA during ZIKV genome replication. Helicase performs ATP hydrolysis to generate energy. The phosphorus atom of the γ-phosphate group of the ATP molecule is attacked by water molecule; then, the γ-phosphate group is released with ADP. With the use of the energy derived from ATP hydrolysis, helicase translocates along nucleic acid strands, unwinds the double-stranded RNA genome.
Moreover, helicase is responsible for hydrolyzing the triphosphate at the 5′ end of RNA, Removes 1 phosphate group at the 5′ end of the RNA, which is an essential step for viral RNA replication (14–16). It then provides conditions for the polymerization of RNA by an RNA-dependent RNA polymerase and the methylation of RNA by methyltransferase, which is crucial for ZIKV replication (13). In addition, it has been reported that the ATPase activity could alter viral replicative capacity and efficiency and consequently change the host innate immune response (17, 18).
Of these processes, ATP hydrolysis represents the most basic event; however, its dynamic mechanisms remain largely unknown, impeding the further understanding of the function of ZIKV helicase.
Disease
Relevance
Structural highlights
5y6n is a 1 chain protein of 438 residues. There are 2 main activities: ATP binding and helicase activity Helicase from Zika virus with a specific domain : a serine protease. It is a specific class of protein that hydrolysis peptide bonds from proteins.
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.
</StructureSection>