We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
LdtMt2
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
[[Image:Figure transpeptidases.png]] | [[Image:Figure transpeptidases.png]] | ||
| - | |||
== Function == | == Function == | ||
| Line 16: | Line 15: | ||
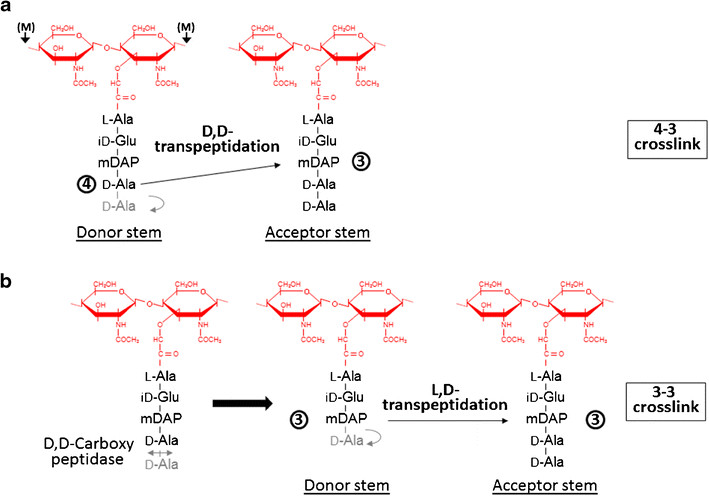
During the stationary phase of growth in bacteria, formation of new 4->3 linkages is impaired because the mature peptidoglycan layer has few pentapeptide stems, so the synthesis of crosslinking is significantly reduced. In contrast, L,D transpeptidases could still crosslink the available tetrapeptide donors. Expression of the LdtMt is upregulated in the transcriptome of laboratory models of persisting bacteria. So the L,D transpeptidase activity could be a chemotherapeutic target. | During the stationary phase of growth in bacteria, formation of new 4->3 linkages is impaired because the mature peptidoglycan layer has few pentapeptide stems, so the synthesis of crosslinking is significantly reduced. In contrast, L,D transpeptidases could still crosslink the available tetrapeptide donors. Expression of the LdtMt is upregulated in the transcriptome of laboratory models of persisting bacteria. So the L,D transpeptidase activity could be a chemotherapeutic target. | ||
| - | |||
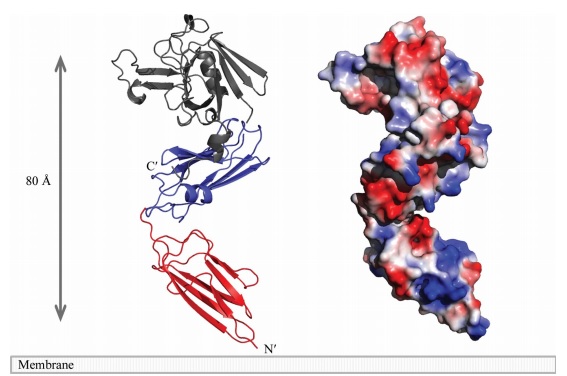
== Structure == | == Structure == | ||
| Line 40: | Line 38: | ||
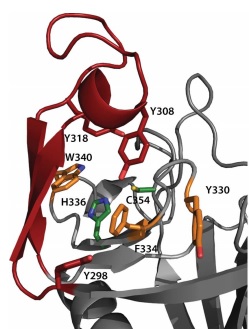
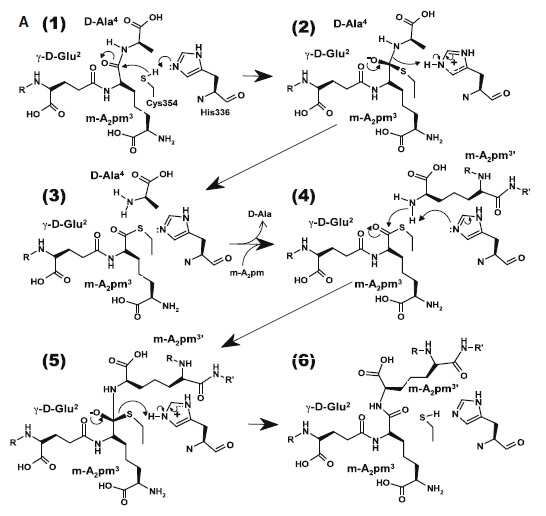
At one end of the tunnel <scene name='81/817533/D-chiral_center_recognition/2'>Tyr318 and the carbonyl group of Gly332</scene> recognize the D chiral center of the meso-diaminopimelic acid side chain, making H bonds to its carboxylate and amide group. At the other end, the L chiral center is surrounded by an <scene name='81/817533/Anion_hole/1'>anion hole</scene> formed by the backbone NH group of residues 352–354 at the C terminus of loop Lc. In this position, the acyl group of the m-DAP L chiral center is within reach of Cys354. '''His336''' is close and ready to accept a H+ from '''Cys354''' (<scene name='81/817533/Conserved_residues/1'>conserved residues</scene>). The cysteine thiolate formed by this H+ abstraction attacks the acyl carbon and forms a tetrahedral intermediate stabilized by the ‘‘anion hole’’ and by the just protonated '''His336'''. After the intermediate thioester formation and protonation by '''His336''', D-Ala4 is released. Then another peptide stem can enter the catalytic site and bind to active site residues with the side-chain amide of the mDAP residue. Nucleophilic attack by this amine group forms the new peptide bond that crosslinks the two stems with release and protonation of the cysteine thiolate by '''His336'''. The last step in the transpeptidation reaction requires that donor and acceptor peptidoglycan stems both be at the catalytic site for the reaction to occur. | At one end of the tunnel <scene name='81/817533/D-chiral_center_recognition/2'>Tyr318 and the carbonyl group of Gly332</scene> recognize the D chiral center of the meso-diaminopimelic acid side chain, making H bonds to its carboxylate and amide group. At the other end, the L chiral center is surrounded by an <scene name='81/817533/Anion_hole/1'>anion hole</scene> formed by the backbone NH group of residues 352–354 at the C terminus of loop Lc. In this position, the acyl group of the m-DAP L chiral center is within reach of Cys354. '''His336''' is close and ready to accept a H+ from '''Cys354''' (<scene name='81/817533/Conserved_residues/1'>conserved residues</scene>). The cysteine thiolate formed by this H+ abstraction attacks the acyl carbon and forms a tetrahedral intermediate stabilized by the ‘‘anion hole’’ and by the just protonated '''His336'''. After the intermediate thioester formation and protonation by '''His336''', D-Ala4 is released. Then another peptide stem can enter the catalytic site and bind to active site residues with the side-chain amide of the mDAP residue. Nucleophilic attack by this amine group forms the new peptide bond that crosslinks the two stems with release and protonation of the cysteine thiolate by '''His336'''. The last step in the transpeptidation reaction requires that donor and acceptor peptidoglycan stems both be at the catalytic site for the reaction to occur. | ||
| + | ==3D structures of ferredoxin== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | + | *L,D-transpeptidase | |
| + | **[[3zqd]] – BsLDT – ''Bacillus subtilis'' - NMR<br /> | ||
| + | **[[4lzh]] – LDT – ''Klebsiella pneumoniae''<br /> | ||
| + | **[[4xvo]] – LDT – ''Mycobacterium smegmatis''<br /> | ||
| + | **[[1zat]] – EfLDT C-terminal 217-466 – ''Enterococcus faecium''<br /> | ||
| + | **[[2hkl]] – EfLDT C-terminal 217-466 (mutant) <br /> | ||
| + | **[[3zg4]] – EfLDT catalytic domain 340-465 - NMR<br /> | ||
| + | **[[5bmq]] – LDT – ''Stackebbrandtia nassauensis''<br /> | ||
| + | **[[5uwv]] – LDT 1 – ''Mycobacterium abscessus''<br /> | ||
| + | **[[4lpq]] – LDT residues 123-326 – ''Xylanimonas cellulosilytica''<br /> | ||
| + | **[[5e5l]], [[4k73]], [[4jmn]] – MtLDT 1 A and B domain residues 32-251 – ''Mycobacterium tuberculosis''<br /> | ||
| + | **[[4qra]] – MtLDT 1 residues 55-408<br /> | ||
| + | **[[4hu2]] – MtLDT 2 A and B domain<br /> | ||
| + | **[[3vyo]], [[4huc]], [[3tur]], [[3u1p]], [[3u1q]], [[3vae]] – MtLDT 2 B and C domain residues 140-408<br /> | ||
| + | **[[3tx4]] – MtLDT 2 B and C domain (mutant)<br /> | ||
| + | **5d7h]], [[5du7]] – MtLDT 2 residues 56-407<br /> | ||
| + | **[6d4k]] – MtLDT 3 A and B domain <br /> | ||
| + | **[[4z7a]], [[6d5a]] – MtLDT 5<br /> | ||
| + | |||
| + | *L,D-transpeptidase complex | ||
| + | |||
| + | **[[5e51]], [[4jmx]] – MtLDT 1 A and B domain + antibiotic <br /> | ||
| + | **[[5dvp]], [[5dc2]], [[5dcc]], [[5dzj]], [[5dzp]], [[5e1g]], [[5e1i]], [[5k69]], [[6boi]], [[5duj]] – MtLDT 2 residues 56-408 + antibiotic <br /> | ||
| + | **[[3vyp]], [[4gsq]], [[4gsr]], [[4gsu]], [[6lyv]], [[6lyw]], [[5lbg]] – MtLDT 2 B and C domain + antibiotic<br /> | ||
| + | **[[5lb1]] – MtLDT 2 B and C domain + thionitrobenzoate<br /> | ||
| + | **6d51]] – MtLDT 3 A and B domain + antibiotic<br /> | ||
| + | **4qrb]] – MtLDT 1 residues 55-408 + pyranose derivative<br /> | ||
| + | **4qr7]] – MtLDT 1 residues 55-408 + pyrroliine derivative + pyranose derivative<br /> | ||
| + | **4qtf]] – MtLDT 1 residues 55-408 + sulfanyl derivative + pyranose derivative<br /> | ||
| + | **[[4zfq]] – MtLDT 5 + meropenem<br /> | ||
| + | **[[3zgp]] – EfLDT catalytic domain 340-465 + antibiotic- NMR<br /> | ||
| + | **[[6fj1]] – EfLDT C-terminal + antibiotic- <br /> | ||
| + | **[[2mtz]] – BsLDT + hexamuropeptide - NMR<br /> | ||
| + | }} | ||
| + | == References == | ||
'''Böth, D., Steiner, E. M., Stadler, D., Lindqvist, Y., Schnell, R., & Schneider, G.''' (2013). Structure of LdtMt2, an L, D-transpeptidase from Mycobacterium tuberculosis. Acta Crystallographica Section D: Biological Crystallography, 69(3), 432-441.[https://scripts.iucr.org/cgi-bin/paper?wd5198] | '''Böth, D., Steiner, E. M., Stadler, D., Lindqvist, Y., Schnell, R., & Schneider, G.''' (2013). Structure of LdtMt2, an L, D-transpeptidase from Mycobacterium tuberculosis. Acta Crystallographica Section D: Biological Crystallography, 69(3), 432-441.[https://scripts.iucr.org/cgi-bin/paper?wd5198] | ||
Revision as of 09:30, 14 January 2020
| |||||||||||