Sandbox Reserved 1102
From Proteopedia
| Line 1: | Line 1: | ||
| - | {{Sandbox_ESBS_2019}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | + | <Structure load='Insert PDB code or filename here' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' />{{Sandbox_ESBS_2019}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> |
== 5y6n- Zika virus helicase in complex with ADP == | == 5y6n- Zika virus helicase in complex with ADP == | ||
Revision as of 14:08, 14 January 2020
|
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
Contents |
5y6n- Zika virus helicase in complex with ADP
5y6n is a 1 chain protein structure. It’s the only helicase belongs to zika virus. Zika helicase plays a role
Function
Zika is an double-stranded RNA virus. The genome replication of double-Stranded RNA virus need intervention of helicase.
Protein from Zika consists of a protease domain at its N terminus and a helicase domain at its C terminus (8–10). The multifunctional helicase, belonging to the superfamily 2 (SF2) helicase family, is an essential enzyme involved in the cycles of ATP hydrolysis and behaves as a motor protein in RNA unwinding (11, 12).
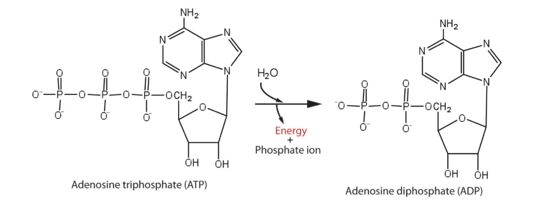
helicase domain that has 5'-triphosphatase activity of Zika performs the critical and indispensable function of unwinding double-stranded RNA during Zika genome replication. Helicase performs ATP hydrolysis at the 5′ end of RNA to generate energy. The phosphorus atom of the γ-phosphate group of the ATP molecule is attacked by water molecule; then, the γ-phosphate group is released with ADP. With the use of the energy derived from ATP hydrolysis, helicase translocates along nucleic acid strands, unwinds the double-stranded RNA genome.
Which is an essential step for viral RNA replication (14–16). It then provides conditions for the polymerization of RNA by an RNA-dependent RNA polymerase and the methylation of RNA by methyltransferase, which is crucial for Zika replication (13). In addition, it has been reported that the ATPase activity could alter viral replicative capacity and efficiency and consequently change the host innate immune response (17, 18).
Of these processes, ATP hydrolysis represents the most basic event; however, its dynamic mechanisms remain largely unknown, impeding the further understanding of the function of Zika helicase.
Disease
Zika virus (ZIKV) is a member of the Flavivirus genus, of the virus family Flaviviridae. The Flavivirus genus comprises the dengue virus (DENV), yellow fever virus, West Nile virus, Japanese encephalitis virus, and tick-borne encephalitis virus. It is transmitted by daytime-active Aedes mosquitoes. Only one over five infected people shows mild symptoms like skin rash, fever ("Zika fever"), joint pain, conjunctivitis and less often muscle pain, headache and vomiting. After a few days, the symptoms subside. However, multiple patients have developed Guillain-Barré-syndrom after a zika virus infection. Infection during first trimester of pregnancy can cause microencephally in newborn children.
Relevance
There is still no vaccines against Zika virus but the study of zika helicase is one of the most promising area for scientific research. It is an enzyme essential for the virulence of Zika virus.
Structural highlights
5y6n is a 1 chain protein of 438 residues. There are 2 main activities: ATP binding and helicase activity. Helicase from Zika virus with a specific domain : a serine protease. It is a specific class of protein that hydrolysis peptide bonds from proteins. The helicase active site has a serine residue that serves as a nucleophilic amino acid. This residue is essential for the catalytic activity of the enzyme.The serine protases present a catalytic triad composed of 3 amino acids His57 Asp102 and Ser195.These 3 amino acids interaction is due to the folding of the protein and allows to cleave the substrate by attacking its carbonyl group. There are two ligands: ADP and Manganese II ion