Sandbox Reserved 1103
From Proteopedia
| Line 1: | Line 1: | ||
| + | ==Your Heading Here (maybe something like 'Structure')== | ||
| + | <Structure load='Insert PDB code or filename here' size='350' frame='true' align='right' caption='Insert caption here' scene='Insert optional scene name here' /> | ||
== Function == | == Function == | ||
| Line 9: | Line 11: | ||
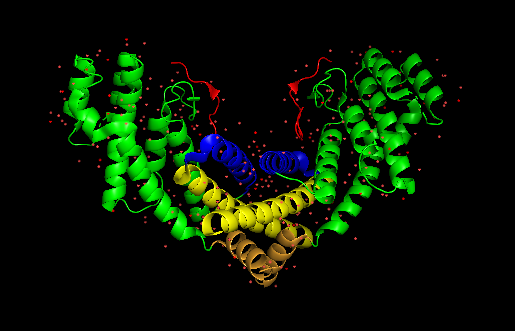
== Structure == | == Structure == | ||
| + | <Structure load='Dimer2.pdb' size='350' frame='false' align='centre' scene='82/829356/Dimer/3'>''3BQO TRF1 TRFH domain and TIN2 peptide complex''' | ||
The TRF1 TRFH domain is a sequence motif of about 200 amino acids located in the centre of TRF1. It is entirely constituted of α helices and binds to another TRF1 TRFH to form a <scene name='82/829356/Dimer/3'>homodimer</scene>. The two monomers are antiparallel and form a homodimer which is symmetrical. There are three α-helices from each monomer involved in this homodimerization: the helices 1, 2 and 9. To form a dimer, the helix 1 of one monomer comes into contact with helix 1 of the other monomer, its helix 2 does it with the helix 2, and so does the helix 9. The two helices 9 stabilize the dimer interface and are perpendicular to the helices 1, forming a cross brace at the top and the bottom of it. The two helices 1 are the core of the dimer interface. This interface involves many hydrophobic interactions and a few hydrogen bonds. The amino acids Trp77 of each helix 1 are central to the formation of the hydrophobic core. “Trp77 packs against Phe255 (helix 9) within the monomer and between Ala259 and Ala260 and against Val263 of helix 9 from its partner” (Fairall L et al Mol Cell.<ref>PMID: 11545737</ref>) The hydrogen bonds involved in the dimer interface are formed between Glu71 of one monomer with Ser85 of the other monomer. Overall, this dimer interface is highly hydrophobic and packed. | The TRF1 TRFH domain is a sequence motif of about 200 amino acids located in the centre of TRF1. It is entirely constituted of α helices and binds to another TRF1 TRFH to form a <scene name='82/829356/Dimer/3'>homodimer</scene>. The two monomers are antiparallel and form a homodimer which is symmetrical. There are three α-helices from each monomer involved in this homodimerization: the helices 1, 2 and 9. To form a dimer, the helix 1 of one monomer comes into contact with helix 1 of the other monomer, its helix 2 does it with the helix 2, and so does the helix 9. The two helices 9 stabilize the dimer interface and are perpendicular to the helices 1, forming a cross brace at the top and the bottom of it. The two helices 1 are the core of the dimer interface. This interface involves many hydrophobic interactions and a few hydrogen bonds. The amino acids Trp77 of each helix 1 are central to the formation of the hydrophobic core. “Trp77 packs against Phe255 (helix 9) within the monomer and between Ala259 and Ala260 and against Val263 of helix 9 from its partner” (Fairall L et al Mol Cell.<ref>PMID: 11545737</ref>) The hydrogen bonds involved in the dimer interface are formed between Glu71 of one monomer with Ser85 of the other monomer. Overall, this dimer interface is highly hydrophobic and packed. | ||
| Line 24: | Line 27: | ||
== Structural highlights == | == Structural highlights == | ||
| - | <Structure load='Dimer2.pdb' size='350' frame='true' align='right' caption='TRF1 TRFH domain and TIN2 peptide complex' scene='82/829356/Dimer/3'>''3BQO TRF1 TRFH domain and TIN2 peptide complex''' | ||
| - | </StructureSection> | ||
== References == | == References == | ||
Revision as of 18:32, 14 January 2020
Your Heading Here (maybe something like 'Structure')
|
Function
TRF1 also called TERF1 (Telomeric repeat-binding factor 1) is a protein part of the Shelterin complex (called also telosome) that has a crucial role in the regulation of telomeres [1]. TRF1 is an inhibitor of Telomerase, the protein that elongate telomeres. Indeed, when TRF1 is inactivated, telomeres are getting longer with no regulation. The TRFH (telomeric repeat factor homology 1h6o) domain is essential to the TRF1 because it’s the sequence where the protein dimerizes to form a functional homodimer. Then, the protein can interact with DNA by fixing to the repeated sequence TTAGGG, and can then remodel DNA. This activity of remodeling is enhanced by the TIN2[2] . TIN2 or TINF2 (TERF1 interacting nuclear factor 2) is also a protein of the Shelterin that can bind to TRF1. It acts as a bridge or a link between TRF1 and TPP or TRF2. This link will regulated their activity and can also stabilize TRF1’s interaction with DNA. When TIN2 is mutated, telomeres are no longer regulated. TRF1 alone doesn’t seems to be efficient to regulate Telomerase. Because of their function in telomeres regulation, TRF1 TIN2 are key proteins involved in cell aging and their dysfunction can directly leads to disease like cancer or other cell cycle diseases.
Structure
| |||||||||||