We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1102
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
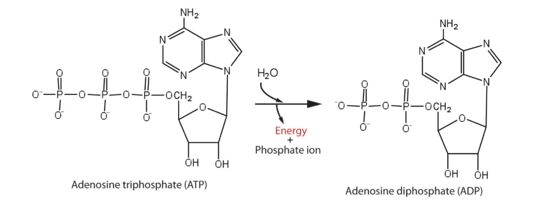
This is an essential step for viral RNA replication. It then provides conditions for the polymerization of RNA by an RNA-dependent RNA polymerase and the methylation of RNA by methyltransferase, which is crucial for Zika replication. In addition, it has been reported that the ATPase activity could alter viral replicative capacity and efficiency and consequently change the host innate immune response. | This is an essential step for viral RNA replication. It then provides conditions for the polymerization of RNA by an RNA-dependent RNA polymerase and the methylation of RNA by methyltransferase, which is crucial for Zika replication. In addition, it has been reported that the ATPase activity could alter viral replicative capacity and efficiency and consequently change the host innate immune response. | ||
| - | ATP hydrolysis is the most basic event to the function of this helicase; however, the others dynamic mechanisms remain largely unknown, impeding the further understanding of the function of Zika helicase. <ref> Mechanism of ATP hydrolysis by the Zika virus helicase, The Faseb journal, 27 sep 2018, X. Yang and H. Yang conceived of and designed the experiments; X. Yang, H. Tian, H. Chi, Z. Mu, and T. Zhang performed the experiments; C. Chen, K. Yang, Q. Zhao, Z. Wang, X. Ji, and H. Yang analyzed the data; and X. Yang, C. Chen, X. Liu, and H. Yang wrote the paper.; https://www.fasebj.org/doi/10.1096/fj.201701140R </ref> | + | ATP hydrolysis is the most basic event to the function of this helicase; however, the others dynamic mechanisms remain largely unknown, impeding the further understanding of the function of Zika helicase. <ref> Mechanism of ATP hydrolysis by the Zika virus helicase, The Faseb journal, 27 sep 2018, X. Yang and H. Yang conceived of and designed the experiments; X. Yang, H. Tian, H. Chi, Z. Mu, and T. Zhang performed the experiments; C. Chen, K. Yang, Q. Zhao, Z. Wang, X. Ji, and H. Yang analyzed the data; and X. Yang, C. Chen, X. Liu, and H. Yang wrote the paper.; https://www.fasebj.org/doi/10.1096/fj.201701140R </ref> |
== Structural highlights == | == Structural highlights == | ||
Revision as of 09:50, 16 January 2020
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
5y6n- Zika virus helicase in complex with ADP
5y6n is a 1 chain protein structure. It’s the only helicase that belongs to zika virus. Zika helicase plays an important role in the pathogenocity of this virus.
| |||||||||||