We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1095
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
AT1 receptor consists of a 376 amino acid string <ref> [http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=4zud&template=main.html Protein Database (PDBsum): 4zud. European Bioinformatics (EBI); 2013.]</ref>. The protein is composed of | AT1 receptor consists of a 376 amino acid string <ref> [http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=4zud&template=main.html Protein Database (PDBsum): 4zud. European Bioinformatics (EBI); 2013.]</ref>. The protein is composed of | ||
<scene name='82/829348/Helix_a/1'>18 α helix</scene> | <scene name='82/829348/Helix_a/1'>18 α helix</scene> | ||
| - | and <scene name='82/829348/B_sheet/1'>3 β sheets</scene>. Moreover, 7 α helixes are made of a majority of hydrophobic amino acids. These helixes are long enough to cross the membrane and create | + | and <scene name='82/829348/B_sheet/1'>3 β sheets</scene>. Moreover, 7 α helixes are made of a majority of hydrophobic amino acids. These helixes are long enough to cross the membrane and create a <scene name='82/829348/Transmambrane_protein/1'>hydrophobic domain</scene> which is situated into the membrane. The human angiotensin receptor is therefore an α helical trans-membrane protein. |
Since the angiotensin receptor belongs to the GPCRs family, those 7 α helixes contain 3 extracellular and 3 intracellular loops. The N terminus corresponds to the extracellular domain. The C terminal domain is located intracellularly. | Since the angiotensin receptor belongs to the GPCRs family, those 7 α helixes contain 3 extracellular and 3 intracellular loops. The N terminus corresponds to the extracellular domain. The C terminal domain is located intracellularly. | ||
=== Ligand binding pocket === | === Ligand binding pocket === | ||
| - | In the extracellular environment, there is a β-hairpin in conjugation with <scene name='82/829348/Disulfuric_bridge/1'>two extracellular disulfure bridges</scene>. This structure is responsible for the opening and the locking of the ligand binding pocket <ref> PMID: 23386604 </ref>. The ligand goes into | + | In the extracellular environment, there is a β-hairpin in conjugation with <scene name='82/829348/Disulfuric_bridge/1'>two extracellular disulfure bridges</scene>. This structure is responsible for the opening and the locking of the ligand binding pocket <ref> PMID: 23386604 </ref>. The ligand goes into a <scene name='82/829348/Ligand_blinding_pocket/1'>hydrophilic pocket</scene> created into the membrane thanks to the 7 α helix which creates a gate between the membrane and the extracellular environment. |
=== G protein-binding site === | === G protein-binding site === | ||
| Line 38: | Line 38: | ||
When the angiotensin II binds to the angiotensin receptor in the ligand binding pocket, the conformation of the trans-membrane domain changes to create a cytosolic cleft for the binding and activation of G proteins. In this cleft, several conserved residues can be found, which form functional motifs present in all [[GPCRs]] <ref> PMID:30608150 </ref>. | When the angiotensin II binds to the angiotensin receptor in the ligand binding pocket, the conformation of the trans-membrane domain changes to create a cytosolic cleft for the binding and activation of G proteins. In this cleft, several conserved residues can be found, which form functional motifs present in all [[GPCRs]] <ref> PMID:30608150 </ref>. | ||
| - | AngII mediates AT1 receptor activation via stacking interactions between Phe8(AngII)/<scene name='82/829348/His_256/2'>His256</scene>(AT1 receptor) and Tyr4(AngII)/<scene name='82/829348/Asn_111/1'>Asn111</scene>(AT1 receptor). This phenomenon results in a conformational change in transmembrane (TM)3-TM6 | + | AngII mediates AT1 receptor activation via stacking interactions between Phe8(AngII)/<scene name='82/829348/His_256/2'>His256</scene>(AT1 receptor) and Tyr4(AngII)/<scene name='82/829348/Asn_111/1'>Asn111</scene>(AT1 receptor). This phenomenon results in a conformational change in transmembrane (TM)3-TM6 helixes and in interaction between TM2 and TM7. |
=== Interaction with drugs === | === Interaction with drugs === | ||
Angiotensin Receptor Blockers (ARBs) are used to cure diseases linked to AT1R. | Angiotensin Receptor Blockers (ARBs) are used to cure diseases linked to AT1R. | ||
| - | The ARB [https://en.wikipedia.org/wiki/Olmesartan Olmesartan] anchored to ATR1 by the residues <scene name='82/829348/Tyr35/6'>Tyr 35</scene>, <scene name='82/829348/Trp84/4'>Trp84</scene> and <scene name='82/829348/Arg167/3'>Arg167</scene>. | + | The ARB [https://en.wikipedia.org/wiki/Olmesartan Olmesartan] is anchored to ATR1 by the residues <scene name='82/829348/Tyr35/6'>Tyr 35</scene>, <scene name='82/829348/Trp84/4'>Trp84</scene> and <scene name='82/829348/Arg167/3'>Arg167</scene>. |
Those three amino acids seem to play an important role in the binding of the drug to AT1R, thanks to the formation of extensive networks of hydrogen bonds and salt bridges with the ligand <ref name="Zhang2015"/>. | Those three amino acids seem to play an important role in the binding of the drug to AT1R, thanks to the formation of extensive networks of hydrogen bonds and salt bridges with the ligand <ref name="Zhang2015"/>. | ||
| Line 50: | Line 50: | ||
=== Interaction with other GPCRs === | === Interaction with other GPCRs === | ||
| - | It has been discovered that AT1Rs were also able to bind with other GPCRs to form homo- or | + | It has been discovered that AT1Rs were also able to bind with other GPCRs to form homo- or hetero-dimers. Those interactions can modify the sensitivity of the receptor, which leads to different physiological and pathological conditions than the GPCR monomer <ref name="Zhang2015"/> <ref name="Takanobu2017">PMID:28648738 </ref>. The most known heterodimers including AT1 receptor are with [[β2 adrenergic receptor]], [https://en.wikipedia.org/wiki/Apelin_receptor the apelin receptor] ([[5vbl]]), and AT2 receptor. Those interactions could be facilitated by several transmembrane domains. |
The oligomeric complexes' formation complicate the understanding of AT1R pharmacology. | The oligomeric complexes' formation complicate the understanding of AT1R pharmacology. | ||
| Line 56: | Line 56: | ||
== Application in the therapeutic field == | == Application in the therapeutic field == | ||
| - | Since angiotensin receptor is involved in the [https://en.wikipedia.org/wiki/Renin%E2%80%93angiotensin_system renin-angiotenisin system], it represents a target of choice to cure some diseases like [https://en.wikipedia.org/wiki/Hypertension hypertension] or [https://en.wikipedia.org/wiki/Heart_failure heart failure]. | + | Since the angiotensin receptor is involved in the [https://en.wikipedia.org/wiki/Renin%E2%80%93angiotensin_system renin-angiotenisin system], it represents a target of choice to cure some diseases like [https://en.wikipedia.org/wiki/Hypertension hypertension] or [https://en.wikipedia.org/wiki/Heart_failure heart failure]. |
An over-stimulation of this receptor seems to be involved in hypertension, coronary artery disease, cardiac hypertrophy, heart failure, arrhythmia, stroke, diabetic nephropathy and ischemic heart and renal diseases <ref name="Takanobu2017"/>. | An over-stimulation of this receptor seems to be involved in hypertension, coronary artery disease, cardiac hypertrophy, heart failure, arrhythmia, stroke, diabetic nephropathy and ischemic heart and renal diseases <ref name="Takanobu2017"/>. | ||
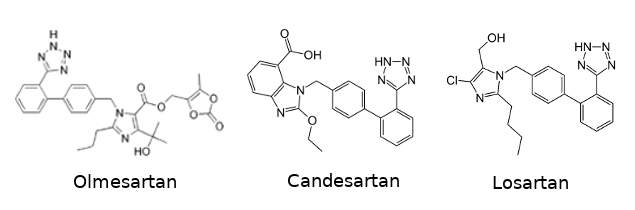
| - | Several anti-hypertensive drugs are targeting the angiotensin receptor in order to block it. | + | Several anti-hypertensive drugs are targeting the angiotensin receptor in order to block it. These kind of drugs are called [https://en.wikipedia.org/wiki/Angiotensin_II_receptor_blocker angiotensin receptor blockers (ARBs)]. This category includes [https://en.wikipedia.org/wiki/Olmesartan olmesartan], [https://en.wikipedia.org/wiki/Candesartan candesartan] and [https://en.wikipedia.org/wiki/Losartan losartan]. One of the common characteristic they share is their biphenyl-tetrazole scaffold. |
[[Image:Sartan_drugs.png]] | [[Image:Sartan_drugs.png]] | ||
Revision as of 17:00, 16 January 2020
| This Sandbox is Reserved from 25/11/2019, through 30/9/2020 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1091 through Sandbox Reserved 1115. |
To get started:
More help: Help:Editing |
Human Angiotensin Receptor
Angiotensin receptors of type 1 belong to the G protein coupled receptor (GPCR) family. These transmembrane proteins interact with angiotensin II, their ligand, and play a crucial role in the renin-angiotensin-aldosterone system. AT1 receptors are predominantly expressed in cardiovascular tissues including heart, endothelium, kidney, vascular smooth muscle cells as well as lungs, brain and adrenal cortex. [1] They are therefore important for the cardiovascular physiology.
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Thomas WG, Mendelsohn FA. Angiotensin receptors: form and function and distribution. Int J Biochem Cell Biol. 2003 Jun;35(6):774-9. doi:, 10.1016/s1357-2725(02)00263-7. PMID:12676163 doi:http://dx.doi.org/10.1016/s1357-2725(02)00263-7
- ↑ Kawai T, Forrester SJ, O'Brien S, Baggett A, Rizzo V, Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol Res. 2017 Nov;125(Pt A):4-13. doi: 10.1016/j.phrs.2017.05.008. Epub, 2017 May 17. PMID:28527699 doi:http://dx.doi.org/10.1016/j.phrs.2017.05.008
- ↑ Goodfriend TL. Angiotensin receptors: history and mysteries. Am J Hypertens. 2000 Apr;13(4 Pt 1):442-9. doi: 10.1016/s0895-7061(99)00212-5. PMID:10821350 doi:http://dx.doi.org/10.1016/s0895-7061(99)00212-5
- ↑ Bumpus FM, Catt KJ, Chiu AT, DeGasparo M, Goodfriend T, Husain A, Peach MJ, Taylor DG Jr, Timmermans PB. Nomenclature for angiotensin receptors. A report of the Nomenclature Committee of the Council for High Blood Pressure Research. Hypertension. 1991 May;17(5):720-1. doi: 10.1161/01.hyp.17.5.720. PMID:2022414 doi:http://dx.doi.org/10.1161/01.hyp.17.5.720
- ↑ Zhang H, Unal H, Desnoyer R, Han GW, Patel N, Katritch V, Karnik SS, Cherezov V, Stevens RC. Structural Basis for Ligand Recognition and Functional Selectivity at Angiotensin Receptor. J Biol Chem. 2015 Sep 29. pii: jbc.M115.689000. PMID:26420482 doi:http://dx.doi.org/10.1074/jbc.M115.689000
- ↑ Zhang H, Unal H, Gati C, Han GW, Liu W, Zatsepin NA, James D, Wang D, Nelson G, Weierstall U, Sawaya MR, Xu Q, Messerschmidt M, Williams GJ, Boutet S, Yefanov OM, White TA, Wang C, Ishchenko A, Tirupula KC, Desnoyer R, Coe J, Conrad CE, Fromme P, Stevens RC, Katritch V, Karnik SS, Cherezov V. Structure of the Angiotensin receptor revealed by serial femtosecond crystallography. Cell. 2015 May 7;161(4):833-44. doi: 10.1016/j.cell.2015.04.011. Epub 2015 Apr, 23. PMID:25913193 doi:http://dx.doi.org/10.1016/j.cell.2015.04.011
- ↑ Protein Database (PDBsum): 4zud. European Bioinformatics (EBI); 2013.
- ↑ Fillion D, Cabana J, Guillemette G, Leduc R, Lavigne P, Escher E. Structure of the human angiotensin II type 1 (AT1) receptor bound to angiotensin II from multiple chemoselective photoprobe contacts reveals a unique peptide binding mode. J Biol Chem. 2013 Mar 22;288(12):8187-97. doi: 10.1074/jbc.M112.442053. Epub 2013, Feb 5. PMID:23386604 doi:http://dx.doi.org/10.1074/jbc.M112.442053
- ↑ Singh KD, Unal H, Desnoyer R, Karnik SS. Mechanism of Hormone Peptide Activation of a GPCR: Angiotensin II Activated State of AT1R Initiated by van der Waals Attraction. J Chem Inf Model. 2019 Jan 28;59(1):373-385. doi: 10.1021/acs.jcim.8b00583. Epub , 2019 Jan 16. PMID:30608150 doi:http://dx.doi.org/10.1021/acs.jcim.8b00583
- ↑ 10.0 10.1 Takezako T, Unal H, Karnik SS, Node K. Current topics in angiotensin II type 1 receptor research: Focus on inverse agonism, receptor dimerization and biased agonism. Pharmacol Res. 2017 Sep;123:40-50. doi: 10.1016/j.phrs.2017.06.013. Epub 2017 Jun, 23. PMID:28648738 doi:http://dx.doi.org/10.1016/j.phrs.2017.06.013