Succinyl-CoA synthetase
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
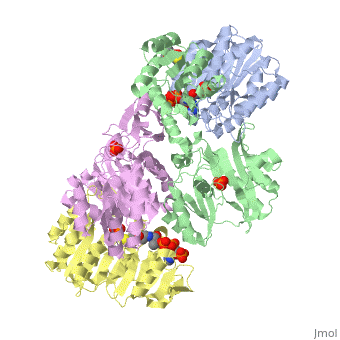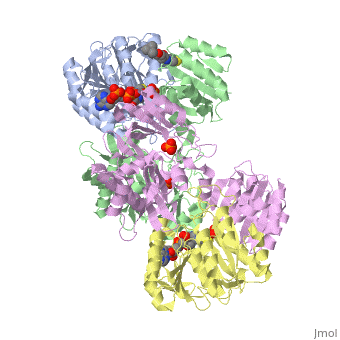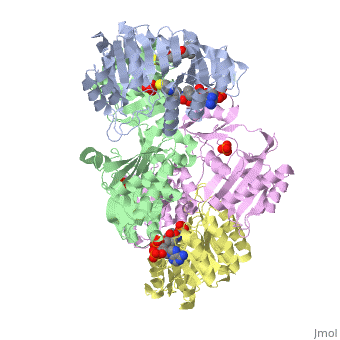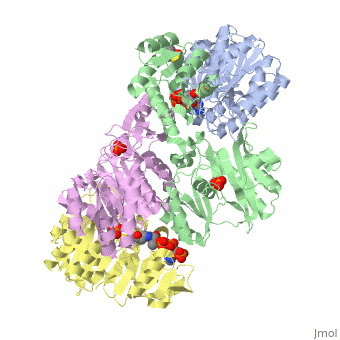
<StructureSection load='1cqj' size='350' side='right' scene='' caption='E. coli succinyl-CoA synthetase α subunit (grey, yellow) and β subunit (green, magenta) complex with CoA and phosphate (PDB code [[1cqj]])'> | <StructureSection load='1cqj' size='350' side='right' scene='' caption='E. coli succinyl-CoA synthetase α subunit (grey, yellow) and β subunit (green, magenta) complex with CoA and phosphate (PDB code [[1cqj]])'> | ||
| + | |||
| + | __TOC__ | ||
| + | ==Function== | ||
'''Succinyl-CoA synthetase''' or '''succinate-CoA ligase''' catalyzes the reversible reaction of succinyl-CoA + NDP + Pi <-> succinate + CoA + NTP (where N is either adenosine or guanosine. It can be found in Escherichia coli and is the fifth step in the [[Citric Acid Cycle|citric acid cycle]]. Due to its involvement in the citric acid cycle it is very important in all living cells that use oxygen as a part of cellular respiration. This entire process (Citric acid cycle) is necessary in producing one GTP or ATP, three NADHs, and two carbon dioxides. See also:<br /> | '''Succinyl-CoA synthetase''' or '''succinate-CoA ligase''' catalyzes the reversible reaction of succinyl-CoA + NDP + Pi <-> succinate + CoA + NTP (where N is either adenosine or guanosine. It can be found in Escherichia coli and is the fifth step in the [[Citric Acid Cycle|citric acid cycle]]. Due to its involvement in the citric acid cycle it is very important in all living cells that use oxygen as a part of cellular respiration. This entire process (Citric acid cycle) is necessary in producing one GTP or ATP, three NADHs, and two carbon dioxides. See also:<br /> | ||
| Line 29: | Line 32: | ||
==Regulation== | ==Regulation== | ||
Succinyl-CoA synthetase is not a major regulator in the Krebs cycle, making it dependent on the steps prior. However, there has been evidence that a high-affinity GDP-binding site does allosterically regulate the activity of the enzyme. It has also been seen that the binding of GDP to these allosteric sites actually leads to an increase in the rate of phosphorylation of Succinyl-Coa synthetase. This could be due to the fact that GDP is probably altering the affinity of the enzyme for GTP.<ref>Um, H.-D., and C. Klein. 1993. Evidence for allosteric regulation of succinyl-CoA synthetase. Biochem. J. 295:821–826.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1134635/]</ref> | Succinyl-CoA synthetase is not a major regulator in the Krebs cycle, making it dependent on the steps prior. However, there has been evidence that a high-affinity GDP-binding site does allosterically regulate the activity of the enzyme. It has also been seen that the binding of GDP to these allosteric sites actually leads to an increase in the rate of phosphorylation of Succinyl-Coa synthetase. This could be due to the fact that GDP is probably altering the affinity of the enzyme for GTP.<ref>Um, H.-D., and C. Klein. 1993. Evidence for allosteric regulation of succinyl-CoA synthetase. Biochem. J. 295:821–826.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1134635/]</ref> | ||
| + | |||
| + | ==3D structures of succinyl-CoA synthetase== | ||
| + | [[Succinyl-CoA synthetase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
| - | + | ||
==3D structures of succinyl-CoA synthetase== | ==3D structures of succinyl-CoA synthetase== | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| Line 37: | Line 44: | ||
*Histidine-phosphorylated succinyl-CoA synthetase | *Histidine-phosphorylated succinyl-CoA synthetase | ||
| - | **[[ | + | **[[6g4q]] - hSCS α+β subunits - human<br /> |
| - | + | ||
**[[1eud]] – pSCS α+β (mutant) subunit - pig<br /> | **[[1eud]] – pSCS α+β (mutant) subunit - pig<br /> | ||
**[[2fp4]] - pSCS α+β subunit + Mg + GTP<br /> | **[[2fp4]] - pSCS α+β subunit + Mg + GTP<br /> | ||
**[[2fpi]], [[2fpp]] - pSCS α+β subunit<br /> | **[[2fpi]], [[2fpp]] - pSCS α+β subunit<br /> | ||
| - | **[[ | + | **[[1scu]], [[2scu]] – EcSCS α+β subunit – ''Escherichia coli''<br /> |
| + | **[[2nu6]], [[2nu7]], [[2nu8]], [[2nu9]], [[2nua]] - EcSCS α (mutant) + β subunit<br /> | ||
*Dephosphorylated succinyl-CoA synthetase | *Dephosphorylated succinyl-CoA synthetase | ||
| - | **[[1cqi]] - EcSCS α+β subunits + Mg + ADP + PO4<br /> | ||
| - | **[[1cqj]], [[1jkj]] - EcSCS α+β subunits + PO4<br /> | ||
| - | **[[1jll]] - EcSCS α+β (mutant) subunits + PO4<br /> | ||
**[[1euc]] – pSCS α+β (mutant) subunits + PO4<br /> | **[[1euc]] – pSCS α+β (mutant) subunits + PO4<br /> | ||
**[[2fpg]] – pSCS α+β subunits + PO4 + GDP<br /> | **[[2fpg]] – pSCS α+β subunits + PO4 + GDP<br /> | ||
**[[4xx0]] - pSCS α+β subunit + PO4 + CoA <br /> | **[[4xx0]] - pSCS α+β subunit + PO4 + CoA <br /> | ||
**[[5cae]] - pSCS α+β subunit + PO4 + CoA + succinate<br /> | **[[5cae]] - pSCS α+β subunit + PO4 + CoA + succinate<br /> | ||
| + | **[[1cqi]] - EcSCS α+β subunits + Mg + ADP + PO4<br /> | ||
| + | **[[1cqj]], [[1jkj]] - EcSCS α+β subunits + PO4<br /> | ||
| + | **[[1jll]] - EcSCS α+β (mutant) subunits + PO4<br /> | ||
**[[1oi7]] – SCS α subunit (mutant) – ''Thermos thermophilus''<br /> | **[[1oi7]] – SCS α subunit (mutant) – ''Thermos thermophilus''<br /> | ||
**[[2yv1]] - SCS α subunit – ''Methanocaldococcus jannaschii''<br /> | **[[2yv1]] - SCS α subunit – ''Methanocaldococcus jannaschii''<br /> | ||
| Line 58: | Line 65: | ||
**[[3ufx]] - SCS α+β subunits + Mn + GDP – ''Thermus aquaticus''<br /> | **[[3ufx]] - SCS α+β subunits + Mn + GDP – ''Thermus aquaticus''<br /> | ||
**[[6mel]] - SCS α+β subunits + citrate – ''Campylobacter jejuni''<br /> | **[[6mel]] - SCS α+β subunits + citrate – ''Campylobacter jejuni''<br /> | ||
| + | **[[6mgg]], [[6pfn]] - SCS (mutant) + subunit (mutant) + CoA – ''Francisella tularensis''<br /> | ||
| + | **[[6no0]] - BhSCS subunit + ADP – ''Blastocystis hominis'' <br /> | ||
| + | **[[6no1]], [[6no2]], [[6no3]], [[6no4]], [[6no5]], [[6no6]] - BhSCS subunit (mutant) + ADP <br /> | ||
}} | }} | ||
==Additional Resources== | ==Additional Resources== | ||
Revision as of 08:33, 11 February 2020
| |||||||||||
Contents |
3D structures of succinyl-CoA synthetase
Updated on 11-February-2020
Additional Resources
References
- ↑ Joyce MA, Fraser ME, Brownie ER, James MN, Bridger WA, Wolodko WT. Probing the nucleotide-binding site of Escherichia coli succinyl-CoA synthetase. Biochemistry. 1999 Jun 1;38(22):7273-83. PMID:10353839 doi:10.1021/bi990527s
- ↑ Fraser ME, Joyce MA, Ryan DG, Wolodko WT. Two glutamate residues, Glu 208 alpha and Glu 197 beta, are crucial for phosphorylation and dephosphorylation of the active-site histidine residue in succinyl-CoA synthetase. Biochemistry. 2002 Jan 15;41(2):537-46. PMID:11781092
- ↑ Bild GS, Janson CA, Boyer PD. Subunit interaction during catalysis. ATP modulation of catalytic steps in the succinyl-CoA synthetase reaction. J Biol Chem. 1980 Sep 10;255(17):8109-15. PMID:6997289
- ↑ Mikeladze DG, Matveeva LN, Severin SE. [Reaction mechanism of succinyl CoA synthetase from pigeon thoracic muscle] Biokhimiia. 1978 Aug;43(8):1458-67. PMID:570066
- ↑ Joyce MA, Fraser ME, James MN, Bridger WA, Wolodko WT. ADP-binding site of Escherichia coli succinyl-CoA synthetase revealed by x-ray crystallography. Biochemistry. 2000 Jan 11;39(1):17-25. PMID:10625475
- ↑ Frank J. Moffet and W. A. Bridger. The Kinetics of Succinyl Coenzyme A Synthetase from Escherichia coli : A REACTION WITH A COVALENT ENZYME-SUBSTRATE INTERMEDIATE NOT EXHIBITING "PING-PONG" KINETICS J. Biol. Chem. 1970 245: 2758-2762.[1]
- ↑ Um, H.-D., and C. Klein. 1993. Evidence for allosteric regulation of succinyl-CoA synthetase. Biochem. J. 295:821–826.[2]
Content Donators
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Lucas Hamlow, J.D. McClintic, Wayne Decatur, David Canner, Alexander Berchansky