We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Ona Ambrozaite/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== General Function == | == General Function == | ||
| - | Trichosurin is one of three predominant proteins found in the milk whey of the common brushtail | + | Trichosurin is one of three predominant proteins found in the milk whey of the common brushtail possum ''Trichosurus vulpecula''. It belongs to the lipocalin superfamily and is an extracellular protein (17–25 kDa) that can bind and transport small lipophilic molecules. The binding pocket is a crucial factor in the selectivity of binding, as is access to the binding pocket that is controlled by side chains of the 22 residues that line the binding pocket and form a loop between β-strands at one end of the lipocalin β-barrel. |
Trichosurin is most closely related to the major urinary proteins (MUPs) from mice and rats and is a significant constituent of milk throughout possum lactation <ref>PMID: 17685895</ref>. | Trichosurin is most closely related to the major urinary proteins (MUPs) from mice and rats and is a significant constituent of milk throughout possum lactation <ref>PMID: 17685895</ref>. | ||
== Structure == | == Structure == | ||
| - | [[Image: | + | |
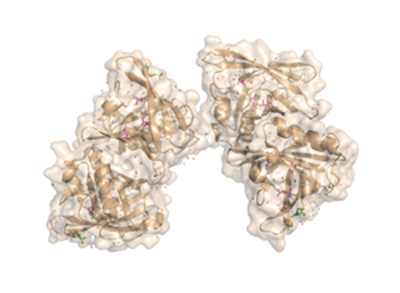
| + | [[Image:Trichosurin_for_ProtPedia.png]] | ||
The fully functional trichosurin consists of two monomers joining to form a dimer of identical eight-stranded, anti-parallel β-barrels. The study of lipocalin dimerization modes has shown six variations in orientation, with trichosurin adding a seventh one to the list. Overall, there are four main chains (A, B, C, and D), in the dimer, with each chain a length of 166 amino acids. There is also a signal sequence at the N-terminus of the chain that is cleaved before a fully functional molecule is formed. Currently, there are two crystal structures of trichosurin that can be found at RCSB, one determined at pH 8.2 and another at a lower pH of 4.6. | The fully functional trichosurin consists of two monomers joining to form a dimer of identical eight-stranded, anti-parallel β-barrels. The study of lipocalin dimerization modes has shown six variations in orientation, with trichosurin adding a seventh one to the list. Overall, there are four main chains (A, B, C, and D), in the dimer, with each chain a length of 166 amino acids. There is also a signal sequence at the N-terminus of the chain that is cleaved before a fully functional molecule is formed. Currently, there are two crystal structures of trichosurin that can be found at RCSB, one determined at pH 8.2 and another at a lower pH of 4.6. | ||
Revision as of 21:37, 6 March 2020
Trichosurin
| |||||||||||