User:Ona Ambrozaite/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
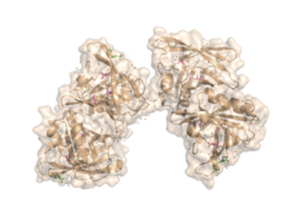
The fully functional trichosurin consists of two monomers joining to form a dimer of identical eight-stranded, anti-parallel β-barrels. The study of lipocalin dimerization modes has shown six variations in orientation, with trichosurin adding a seventh one to the list. Overall, there are four main chains (A, B, C, and D), in the dimer, with each chain a length of 166 amino acids. There is also a signal sequence at the N-terminus of the chain that is cleaved before a fully functional molecule is formed. Currently, there are two crystal structures of trichosurin that can be found at RCSB, one determined at pH 8.2 and another at a lower pH of 4.6. | The fully functional trichosurin consists of two monomers joining to form a dimer of identical eight-stranded, anti-parallel β-barrels. The study of lipocalin dimerization modes has shown six variations in orientation, with trichosurin adding a seventh one to the list. Overall, there are four main chains (A, B, C, and D), in the dimer, with each chain a length of 166 amino acids. There is also a signal sequence at the N-terminus of the chain that is cleaved before a fully functional molecule is formed. Currently, there are two crystal structures of trichosurin that can be found at RCSB, one determined at pH 8.2 and another at a lower pH of 4.6. | ||
| + | With regards to the binding cavity, there are the three asparagine residues, Asn49, Asn97 and Asn127, which lie in the pocket and offer hydrogen-bonding sites for a potential ligand, especially Asn49. | ||
| + | The SCOP website classifies trichosurin as a fatty acid retinoid binding protein, just like the nematode fatty acid retinoid binding protein from ''Necator americanus'' species. Trichosurin belongs to the calycin beta-barrel core domain superfamily, as indicated by the search results on the CATH website, which emphasizes the presence of the β-barrels throughout the structure. | ||
== Structure and Function == | == Structure and Function == | ||
| Line 18: | Line 20: | ||
<Structure load='2RA6' size='350' frame='true' align='right' caption='Crystal Structure of the Possum Milk Whey Lipocalin Trichosurin at pH 4.6 with Bound 4-ethylphenol' scene='Hydrogen Bond Interactions with Ligands' /> | <Structure load='2RA6' size='350' frame='true' align='right' caption='Crystal Structure of the Possum Milk Whey Lipocalin Trichosurin at pH 4.6 with Bound 4-ethylphenol' scene='Hydrogen Bond Interactions with Ligands' /> | ||
| - | The binding site is found in the centre of the β-barrel and can be occupied by water and isopropanol molecules from the corresponding crystallization medium. The protein was also crystallized with 2-naphthol and 4-ethylphenol that were bound in the centre of the β-barrel. The presence of such phenolic compounds provides clues to the function of trichosurin | + | The binding site is found in the centre of the β-barrel and can be occupied by water and isopropanol molecules from the corresponding crystallization medium. The protein was also crystallized with 2-naphthol and 4-ethylphenol that were bound in the centre of the β-barrel. The presence of such phenolic compounds provides clues to the function of trichosurin . |
| - | Overall, trichosurin follows the general lipocalin superfamily fold pattern by having a β-barrel, several extended loop regions between β strands that close the ends of the barrel, a helix near the C-terminus which is common to all lipocalins, and a major conserved feature of the lipocalin fold | + | Overall, trichosurin follows the general lipocalin superfamily fold pattern by having a β-barrel, several extended loop regions between β strands that close the ends of the barrel, a helix near the C-terminus which is common to all lipocalins, and a major conserved feature of the lipocalin fold, a disulfide bond, formed by two cysteines (Cys73 and Cys166 ) in each chain that ties the C-terminus to the corresponding β-strand. |
| - | + | As expected for an extracellular protein, trichosurin displays a large amount of hydrophilic surface area, with the more hydrophobic areas between the helix, the outside of the barrel, and the dimer interface where ligands such as 2-naphthol can bind. | |
Revision as of 23:16, 6 March 2020
Trichosurin
| |||||||||||