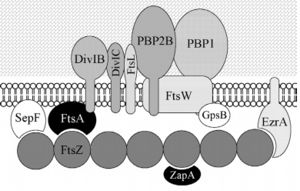
Bacillus subtilis division protein
Bacillus subtilis is a prokaryotic organism, a Gram-positive bacterium, and thus has a cytoplasmic membrane plus a thick cell wall made of peptidoglycan and associated anionic polymers, such as teicoic acid. Bacteria are recognized for the reproductive success and conquest of various environments on Earth, with the presence of a complex and sophisticated machinery of cell division and formation of identical daughter cells a potent factor in this success. There are currently 24 proteins known to be associated with division in B. subtilis: ClpX, DivIB,DivIC, DivIVA, EzrA, FtsA, FtsL, FtsW, FtsZ,GpsB, MciZ, MinC, MinD, MinJ, Noc, PBP1,PBP2B, SepF, SftA, SpoIIE, SpoIIIE, UgtP, YneA and ZapA. These proteins can be divided in two main groups: proteins that make up the divisome - macromolecular complex composed of about 20 proteins, which promotes the construction of the cell wall and cytoplasmic membrane, forming the division septum (DivIB, DivIC, EzrA, FtsA, FtsL, FtsW,FtsZ, GpsB, PBP1, PBP2B, SepF and ZapA) and proteins that regulate the assembly of the divisome (ClpX, DivIVA, MciZ, MinC, MinD, MinJ,Noc, UgtP and YneA).
Among these, the FtsZ stands out for acting in the recruitment of other proteins for the formation of the constriction ring that culminates in cell division.
General Function
FtsZ (Filamentation temperature sensitive Z) is the main coordinator of septum formation and the most widely conserved division protein, being present in essentially all bacterial genomes that have been sequenced to date. In eubacteria, the FtsZ gene is usually found in the dcw gene cluster, a DNA region containing division and cell-wall synthesis gene. This protein is extremely important to binary fission - more specifically, formation of the Z ring - in rod-shaped bacteria entails the formation of a transverse septum that divides a progenitor cell into two equal-sized daughter cells. Septum formation is faithfully coordinated with chromosome replication and segregation and the spatial control, on the other hand, is evident in the placement of the newly formed septum at precisely the middle of the progenitor cell, which ensures that the two daughter cells generated are morphologically and genetically equivalent. It means that despite the apparent simplicity, cell division in bacteria is subject to tight spatiotemporal control.
Structure and Biochemistry of FtsZ
FtsZ is a tubulin-like protein, which is widely conserved in bacteria and the main component of the bacterial cytokinesis machine, or “divisome.” FtsZ is a 40 kDa protein that folds into two independent globular domains [ (1-203) and (204-316)] and has an unstructured tail of about 50 amino acids followed by a 15–17 conserved amino acid sequence at its extreme C-terminus. This conserved terminal sequence is also known as the ‘C-terminal peptide’ (CTP), since it is in the N-terminal domain that the nucleotide binding region is contained. Self-assembly of FtsZ involves interactions between the C-terminal globular domain of one subunit with the N-terminal globular domain of another subunit. The CTP, on the other hand, is the binding site for several of the proteins that interact with FtsZ. The N-terminal and C-terminal domain are separated by the central (178-202).
FtsZ and tubulin share several essential properties: their assembly is cooperative, stimulated by GTP, and leads to GTP hydrolysis; they form dynamic polymers whose turnover is dependent on nucleotide hydrolysis; they use essentially the same bond for polymer formation; and recent evidence indicates that they undergo similar allosteric transitions upon polymerization. The folding of the FtsZ N-terminal domain is typical of GTPases, with six parallel β-strands (S1-S6) surrounded by six α-helices (H1-H6), named according to the tubulin structure (show ). The C-terminal domain is formed by four parallel β-strands (S7-S10) surrounded by two helices, with the antiparallel strand S10. The residues in the T1-T4 loops make contact with the phosphate groups of the GDP. The T5 loop between S5 and H5 helix contains residues that make hydrogen bonds with the sugar moiety and also contacts with the phosphate of GDP, while interactions with the nucleotide nitrogen base are done by residues of the H7 helix.
Polymerization mechanism
In order for polymerization of the monomers of FtsZ to occur, there is a need for interaction of the N-terminal nucleotide binding domain with the C-terminal domain of another monomer. The formation of a complete GTP hydrolysis site depends on the positioning of acid residues of the T7 loop at the nucleotide binding site of the prior monomer in the polymer, explaining why the GTPase activity of FtsZ only occurs when the protein is in the polymer form. (PDB code 1w5b). Water molecules are shown as red spheres. The fact that FtsZ GTPase activity interferes with the dynamics of its autoassociation and the morphology of its polymers, as well as with microtubules and actin, opens the possibility that FtsZ can also present dynamic treadmilling or instability. Dynamic FtsZ beams were observed in vitro and protofilaments exhibiting treadmilling dependent on FtsA protein.
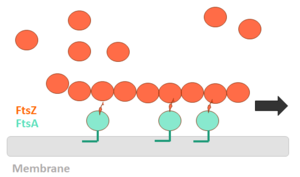
FtsZ polimerization mechanism
The binding of GTP promotes the longitudinal association of the monomers forming protofilaments, and the hydrolysis of the nucleotide leads to depolymerization and consequent disorganization of the protofilament. FtsZ protofilaments have a strong tendency to associate further to form multi-stranded polymers. The tendency of FtsZ filaments to form lateral interactions in greatly increased by the presence of cations such the Ca2+.
However, there is still controversy regarding the mechanism of polymerization of FtsZ and details of the process are being elucidated. What is currently in place is that loop motion between the H6-H7 helices away from the nucleotide cavity (downward movement of the H7 helix, and 23-degree rotation of the C-terminal domain relative to the N-terminal), creates a groove between the C-terminal domain and the H7 helix that does not exist in the structures described above. This conformation promotes the insertion of the T7 loop in the active site of another monomer, which in the presence of divalent cation would stabilize a dimeric interface with more extensive contacts leading to polymerization. Therefore, the "assembly switch" would be the conversion of a monomer into the closed conformation (without the groove between H7 and C-terminal) to the open conformation (with the groove between H7 and the C-terminus), the latter competent for polymerization.
Relevance
The understanding of the structure of FtsZ and its polymerization process is relevant because it can be used as a target for the design of new antibiotics capable of selectively combating bacterial infections. This is due to its structural and functional conservation in prokaryotes, to their essential role in the replication of these organisms, and the evolutionarily distant from tubulin, its counterpart in eukaryotes. Thus, the use of an antibiotic would not cause effects on eukaryotic tubulin.
3D structures of cell division protein
Cell division protein 3D structures