Sandbox Reserved 1600
From Proteopedia
(Difference between revisions)
| Line 12: | Line 12: | ||
=Structure= | =Structure= | ||
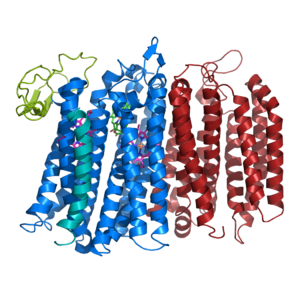
[[Image:Bd oxidase structure (basic).png|300 px|right|thumb|Figure 1. bd oxidase; two structurally similar transmembrane helices in blue and red; CydX subunit in teal; Q loop in lime green. ]] | [[Image:Bd oxidase structure (basic).png|300 px|right|thumb|Figure 1. bd oxidase; two structurally similar transmembrane helices in blue and red; CydX subunit in teal; Q loop in lime green. ]] | ||
| - | The overall structure contains 19 transmembrane helices that are arranged in a nearly oval shape (Fig 1.) | + | The overall structure contains 19 transmembrane helices that are arranged in a nearly oval shape (Fig 1.) <ref name=”Safarian”>PMID:T27126043</ref> The protein contains two structurally similar subunits each containing nine helices (blue and red) and one smaller subunit, CydX, with one transmembrane helix. The subunits are interacting using hydrophobic residues and symmetry at the interfaces. The CydX subunit, whose function is not currently known, is positioned in the same way as CydS, which is found in E. coli bd oxidase. Due to its similar structure and position, it has been hypothesized to potentially stabilize heme b558 during potential structural rearrangements of the Q loop upon binding and oxidation of quinol. The Q loop is shown in lime green, and is a hydrophilic region above Cyd A. The lack of hydrogen bonding in this hydrophobic protein allows the protein to be flexible and go through a large conformational change for reduction of dioxygen. |
==Active Site== | ==Active Site== | ||
Revision as of 18:41, 29 March 2020
bd oxidase; Geobacillus thermodenitrificans
| |||||||||||
References
- ↑ Giuffre A, Borisov VB, Arese M, Sarti P, Forte E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim Biophys Acta. 2014 Jul;1837(7):1178-87. doi:, 10.1016/j.bbabio.2014.01.016. Epub 2014 Jan 31. PMID:24486503 doi:http://dx.doi.org/10.1016/j.bbabio.2014.01.016
- ↑ Safarian S, Hahn A, Mills DJ, Radloff M, Eisinger ML, Nikolaev A, Meier-Credo J, Melin F, Miyoshi H, Gennis RB, Sakamoto J, Langer JD, Hellwig P, Kuhlbrandt W, Michel H. Active site rearrangement and structural divergence in prokaryotic respiratory oxidases. Science. 2019 Oct 4;366(6461):100-104. doi: 10.1126/science.aay0967. PMID:31604309 doi:http://dx.doi.org/10.1126/science.aay0967
- ↑ Junemann S. Cytochrome bd terminal oxidase. Biochim Biophys Acta. 1997 Aug 22;1321(2):107-27. doi:, 10.1016/s0005-2728(97)00046-7. PMID:9332500 doi:http://dx.doi.org/10.1016/s0005-2728(97)00046-7
- ↑ Das A, Silaghi-Dumitrescu R, Ljungdahl LG, Kurtz DM Jr. Cytochrome bd oxidase, oxidative stress, and dioxygen tolerance of the strictly anaerobic bacterium Moorella thermoacetica. J Bacteriol. 2005 Mar;187(6):2020-9. doi: 10.1128/JB.187.6.2020-2029.2005. PMID:15743950 doi:http://dx.doi.org/10.1128/JB.187.6.2020-2029.2005
- ↑ PMID:T27126043