Sandbox Reserved 1627
From Proteopedia
(Difference between revisions)
| Line 8: | Line 8: | ||
==Structural Highlights== | ==Structural Highlights== | ||
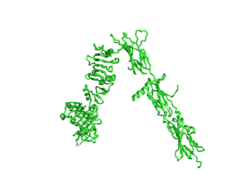
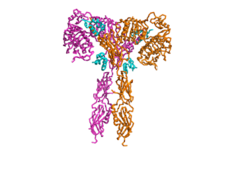
| - | The insulin receptor is a dimer of heterodimers made of two alpha subunits and two beta subunits <ref name=”Tatulian”>PMID:26322622</ref>.The <scene name='83/832953/Alpha_subunits/1'>Alpha | + | The insulin receptor is a dimer of heterodimers made of two alpha subunits and two beta subunits <ref name=”Tatulian”>PMID:26322622</ref>.The <scene name='83/832953/Alpha_subunits/1'>Alpha chains</scene> are on the extracellular side of the membrane and is critical for binding insulin. The <scene name='83/832953/Binding_sites/1'>binding sites</scene> that have the potential to interact with insulin on the extracellular side of the membrane, but it is generally more common for only one or two insulin molecules to bind to the receptor due to the occurrence of negative affinity at the binding site. The <scene name='83/832953/Beta_subunits/1'>Beta chains</scene> are transmembrane subunits that contain a tyrosine kinase region. Beta chains experience a conformation change that brings them from a V shape to a T shape upon activation or binding of an insulin molecule. When the two subunits are brought near to each other in the activated T form, each Tyrosine Kinase region is able to autophosphorylate its counterparts at particular Tyrosine locations. This aspect of the molecule has not yet been imaged. |
== Function == | == Function == | ||
Revision as of 21:47, 29 March 2020
Homo sapiens Insulin Receptor
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39. PMID:16278749
- ↑ Riddle MC. Treatment of diabetes with insulin. From art to science. West J Med. 1983 Jun;138(6):838-46. PMID:6351440
Student Contributors
- Harrison Smith
- Alyssa Ritter