This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1627
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
==Introduction== | ==Introduction== | ||
| - | The insulin receptor is a transmembrane protein receptor that is a vital proponent of cellular function. It plays a key role in a variety of cellular pathways including glucose homeostasis, regulation of lipid, protein, and carbohydrate metabolism, gene expression, and even modulation of brain neurotransmitter levels. '''This page focuses specifically on the insulin receptor's role in glucose homeostasis'''. | + | The insulin receptor is a transmembrane protein receptor that is activated by the binding of insulin and is a vital proponent of cellular function. The insulin receptor belongs to the large class of tyrosine kinase receptors. It plays a key role in a variety of cellular pathways including glucose homeostasis, regulation of lipid, protein, and carbohydrate metabolism, gene expression, and even modulation of brain neurotransmitter levels. '''This page focuses specifically on the insulin receptor's role in glucose homeostasis'''. |
==Structural Highlights== | ==Structural Highlights== | ||
| Line 12: | Line 12: | ||
== Function == | == Function == | ||
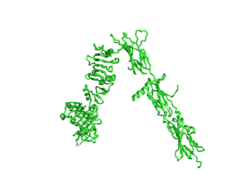
| - | [[Image:Insulin inverted v.png|250px|right|thumb|Figure 1. An image of the inactive Insulin Receptor Alpha subunit in the inverted V conformation. This image shows only a protomer of the alpha subunit because the entire alpha subunit dimer has been unable to be photographed because the transition state has yet to be determined.]] | + | [[Image:Insulin inverted v.png|250px|right|thumb|Figure 1. An image of the inactive Insulin Receptor Alpha subunit in the inverted V conformation. This image shows only a protomer of the inactive alpha subunit because the entire inactive alpha subunit dimer has been unable to be photographed because the transition state has yet to be determined [https://www.rcsb.org/structure/6ce7/ PDB: 6CE7].]] |
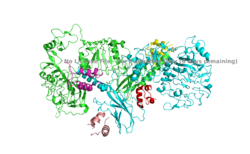
| - | [[Image:Alpha Subunit with Insulin bound.png|250px|right|thumb|Figure 2.]] | + | [[Image:Alpha Subunit with Insulin bound.png|250px|right|thumb|Figure 2. An image of the dimer of an active alpha subunit. The light blue and green areas represent the alpha subunit of the insulin receptor. The magenta, light pink, yellow, and red colorings are the four insulin binding sites with insulin bound. [https://www.rcsb.org/structure/6pxw/ PDB: 6PXW].]] |
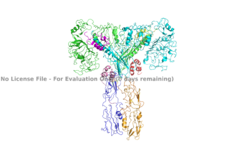
| - | [[Image: | + | [[Image:Insulin receptor domain colored.png|250px|right|thumb|Figure 3. An image of the active insulin receptor showing both the alpha and beta subunits and insulin bound to the four binding sites. The dimers of the alpha subunit are colored in light blue and green. The intracellular beta sites are colored in blue and orange. The insulin binding sites with insulin bound are colored in magenta, light pink, yellow, and red. [https://www.rcsb.org/structure/6sof/ PDB: 6SOF].]] |
| - | + | ||
| - | + | ||
| + | The insulin receptor's function in regards to glucose homeostasis is to begin the signaling pathway that will eventually move glucose transporters to the cell surface which will allow glucose to passively defuse into the cell. The glucose receptor is inactive in the absence of insulin. When insulin does bind to the receptor, it undergoes a conformation change from the inactive inverted V state (Figure 1) to the active T state (Figures 2 and 3). Once activated, the intracellular Beta subunits move together close enough to autophosphorylate, and downstream signaling begins by the phosphorylation of the Insulin Receptor Substrate (IRS). | ||
| + | ===Structure=== | ||
| + | The insulin receptor is a receptor tyrosine kinase that exists in two stable conformations, an inactive and active state. The entire insulin receptor is a dimer of heterodimers with two extracellular alpha subunits, and two transmembrane/intracellular beta subunits linked and stabilized by multiple disulfide bonds. | ||
| + | ======Alpha Subunit====== | ||
| + | The alpha subunit is the extracellular domain of the insulin receptor and is the site of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a C-terminal alpha helix. The actual site of insulin binding occurs at the C-terminal alpha helix and is stabilized by the L1 and L2 domains. | ||
| + | ======Beta Subunit====== | ||
| + | The beta subunit is the membrane spanning and intracellular portion of the insulin receptor. This domain is composed of three Fibronectin domains (FN III-1,-2,-3) and the tyrosine kinase domain. The tyrosine kinase domain is the site of autophosphorylation upon activation of the receptor. | ||
===Conformation Change=== | ===Conformation Change=== | ||
| - | The insulin receptor | + | The inactive form of the insulin receptor predominates in low-levels of circulating insulin, whereas the active conformation is seen when insulin binds to any of the 4 receptor sites. The inactive conformation resembles an inverted V (Figure 1), and the active conformation resembles a T (Figure 2). Upon the binding of insulin to any of the four binding sites, the conformation change will begin, causing the Beta subunit's tyrosine kinase domains to move close together, allowing them to autophosphorylate. This autophosphorylation is what activates the insulin receptor and allows it to participate in further downstream signaling pathways. <ref> DOI 10.1038/s41467-018-06826-6</ref>. |
| + | |||
| + | The insulin receptor can maximally bind four insulins to the active T-state at the four distinct insulin binding sites. Once insulin binds, the structural change from inactive to active state is stabilized by a tripartite interaction between insulin, L1, the C-terminal alpha helix, and the FNIII-1 domain. Studies have found that optimal insulin receptor activation requires the binding of multiple insulin ligands to two insulin binding sites. In (Figure 3) these two binding sites are colored in magenta and red. Binding of at least one insulin to the red binding site in (Figure 3) is required for the activation of the insulin receptor and the change in conformation to the active T state. <ref> DOI 10.7554/eLife.48630 </ref>. | ||
| + | |||
<scene name='83/832953/Alpha_c_helix/1'>α-CT chain</scene> | <scene name='83/832953/Alpha_c_helix/1'>α-CT chain</scene> | ||
| Line 28: | Line 36: | ||
As mentioned in the Introduction, the insulin receptor is relevant to numerous biological functions of the body. In a healthy, normal-functioning human, each cell has many insulin receptors that reacts to insulin when blood glucose levels rise. Without properly functioning insulin receptors, medical intervention is necessary for survival. | As mentioned in the Introduction, the insulin receptor is relevant to numerous biological functions of the body. In a healthy, normal-functioning human, each cell has many insulin receptors that reacts to insulin when blood glucose levels rise. Without properly functioning insulin receptors, medical intervention is necessary for survival. | ||
=== Disease === | === Disease === | ||
| - | One of the most common diseases involving the insulin receptor is diabetes mellitus. There are two types of diabetes- which are referred to as type 1 and type 2 diabetes. Type 1 diabetes is classified as "insulin dependent" and is characterized by an inability for the body to produce insulin. This is most often the result of damage or insufficiency in the Islets of Langerhans in the pancreas. Type 2 diabetes is classified as "insulin independent" and is the result of the body producing insufficient amounts of insulin, or not responding to the insulin. This often occurs because of high blood-glucose levels. Both types of diabetes are often treated with insulin injections, and diet and lifestyle changes. <ref name="Wilcox"> PMID:16278749</ref> <ref name= "Riddle"> PMID: 6351440</ref>. | + | One of the most common diseases involving the insulin receptor in regards to glucose uptake and homeostasis is diabetes mellitus. There are two types of diabetes- which are referred to as type 1 and type 2 diabetes. Type 1 diabetes is classified as "insulin dependent" and is characterized by an inability for the body to produce insulin. This is most often the result of damage or insufficiency in the Islets of Langerhans in the pancreas. Type 2 diabetes is classified as "insulin independent" and is the result of the body producing insufficient amounts of insulin, or not responding to the insulin. This often occurs because of high blood-glucose levels. Both types of diabetes are often treated with insulin injections, and diet and lifestyle changes. <ref name="Wilcox"> PMID:16278749</ref> <ref name= "Riddle"> PMID: 6351440</ref>. |
[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1010832/ Treatment of Diabetes with Insulin] | [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1010832/ Treatment of Diabetes with Insulin] | ||
Revision as of 22:26, 29 March 2020
Homo sapiens Insulin Receptor
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39. PMID:16278749
- ↑ Riddle MC. Treatment of diabetes with insulin. From art to science. West J Med. 1983 Jun;138(6):838-46. PMID:6351440
Student Contributors
- Harrison Smith
- Alyssa Ritter