This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 1: | Line 1: | ||
='''Gamma Secretase'''= | ='''Gamma Secretase'''= | ||
| - | + | <StructureSection load='5FN2' size='350' frame='true' side='right' caption='Human Gamma Secretase Basic Structure' scene=’’> | |
| - | <StructureSection load=' | + | |
=Gamma Secretase= | =Gamma Secretase= | ||
| Line 27: | Line 27: | ||
[[Image:Asp with Pal labeled.png|300 px|right|thumb|Active Site of Gamma Secretase]] | [[Image:Asp with Pal labeled.png|300 px|right|thumb|Active Site of Gamma Secretase]] | ||
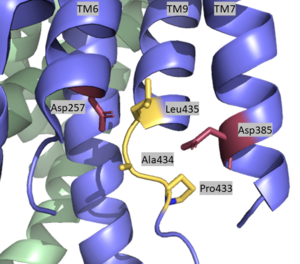
The active site is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Each of these transmembrane helices has an aspartate residue, Asp257 and Asp385, which are located approximately 10.6 A˚ apart when inactive.<ref name="Bai">PMID:26280335</ref> The PAL sequence of Pro433, Ala434, and Leu435 is in close proximity with the catalytic aspartates and is important to substrate recognition. Gamma secretase becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate and the two Asp residues hydrogen bond to each other during catalysis. Asp257 and Asp385 are located 6–7 Å away from the scissile peptide bond of the substrate. | The active site is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Each of these transmembrane helices has an aspartate residue, Asp257 and Asp385, which are located approximately 10.6 A˚ apart when inactive.<ref name="Bai">PMID:26280335</ref> The PAL sequence of Pro433, Ala434, and Leu435 is in close proximity with the catalytic aspartates and is important to substrate recognition. Gamma secretase becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate and the two Asp residues hydrogen bond to each other during catalysis. Asp257 and Asp385 are located 6–7 Å away from the scissile peptide bond of the substrate. | ||
| + | |||
==Relevance== | ==Relevance== | ||
| Line 36: | Line 37: | ||
Important to look at differences between substrates | Important to look at differences between substrates | ||
| - | |||
| + | </StructureSection> | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 00:01, 30 March 2020
Gamma Secretase
| |||||||||||
References
- ↑ Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
Student Contributors
Layla Wisser
Daniel Mulawa