We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madison Summers/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 15: | Line 15: | ||
===Transmembrane Domain=== | ===Transmembrane Domain=== | ||
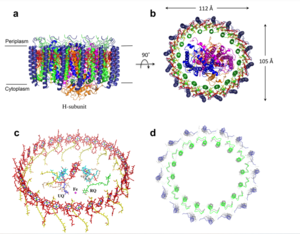
| - | The transmembrane domain is | + | The transmembrane domain is on the inner mitochondrial membrane open to the inner membrane space. The small pore, highly specific for calcium binding is located in the <scene name='83/837230/Transmembrane_domain/1'>transmembrane domain</scene>, in TM2 (transmembrane 2) while TM 1 (transmembrane 1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. The domain swapping of TM1 of one subunit with the TM2 of the neighboring subunits allows for a tight packing in the transmembrane connectivity. It is important that the selectivity pore is small, allowing only a dehydrated calcium molecule to interact with the 5 ampier wide glutamate ring. Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12 ampier wide pore allowing high conductivity. The wider opening allows calcium to rehydrate. |
| - | + | ||
===Coiled coil=== | ===Coiled coil=== | ||
Revision as of 00:15, 2 April 2020
Mitochondrial Calcium Uniporter, E. coli
| |||||||||||
References
- ↑ Ransey E, Paredes E, Dey SK, Das SR, Heroux A, Macbeth MR. Crystal structure of the Entamoeba histolytica RNA lariat debranching enzyme EhDbr1 reveals a catalytic Zn(2+) /Mn(2+) heterobinucleation. FEBS Lett. 2017 Jul;591(13):2003-2010. doi: 10.1002/1873-3468.12677. Epub 2017, Jun 14. PMID:28504306 doi:http://dx.doi.org/10.1002/1873-3468.12677
- ↑ Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018 Jun 28. pii: science.aar4056. doi: 10.1126/science.aar4056. PMID:29954988 doi:http://dx.doi.org/10.1126/science.aar4056
Student Contributors
- Madison Summers
- Holly Rowe
- Lizzy Ratz