Sandbox Reserved 1627
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
===Structure=== | ===Structure=== | ||
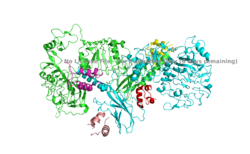
| - | The insulin receptor is a receptor tyrosine kinase that exists in two stable conformations, an inactive and active state. The entire insulin receptor is a dimer of heterodimers with two extracellular alpha subunits, and two transmembrane/intracellular beta subunits linked and stabilized by multiple disulfide bonds. | + | The insulin receptor is a receptor tyrosine kinase that exists in two stable conformations, an inactive and active state. The entire insulin receptor is a dimer of heterodimers with two extracellular alpha subunits, and two transmembrane/intracellular beta subunits linked and stabilized by multiple disulfide bonds. The Cryo-EM structure of the extracellular domain of the Insulin Receptor without the presence of insulin bound at its alpha subunit site was established first, and is also known as the apo-form. The shape that it displayed appeared as an upside down V. Then, a subsequent Cryo-EM was established with insulin bound to the alpha subunit binding site, displaying a T shape conformation (UCHIKAWA). |
| + | |||
======Alpha Subunit====== | ======Alpha Subunit====== | ||
| - | The alpha subunit (Figure 1) is the extracellular domain of the insulin receptor and is the site of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a C-terminal alpha helix. The actual site of insulin binding occurs at the <scene name='83/832953/Alpha_c_helix/1'>α-CT chain</scene> and is stabilized by the L1 and L2 domains <scene name='83/832953/Inactive_insulin_receptor/2'> Not sure what these are sos</scene>. | + | The alpha subunit (Figure 1) is the extracellular domain of the insulin receptor and is the site of insulin binding. The subunit is unqiue in the way it binds insulin, and has four potential sites for binding. Each site performs binding via a "cross link", which is due to the fact that the receptor is a dimer of heterodimers and contains four protomers of similar structure. Each time an insulin ligand binds, the it comes in contact with the L1 domain of one protomer, and the alpha-CT chain and FnIII-1 loop of another protomer. This cross binding can occur in two sites (1 and 1'). There is potential for binding at sites 2 and 2', but it is less likely due to negative cooperation and the location of the sites on the back of the beta sheet of the FnIII-1 domain on each protomer. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a C-terminal alpha helix. The actual site of insulin binding occurs at the <scene name='83/832953/Alpha_c_helix/1'>α-CT chain</scene> and is stabilized by the L1 and L2 domains <scene name='83/832953/Inactive_insulin_receptor/2'> Not sure what these are sos</scene>. The T shape conformation was also well observed in the alpha subunit. It is horizontally composed of L1, CR (including the alpha-CT chain), and L2 domains and vertically composed of the FnIII-1, 2, and 3 domains. |
| + | |||
======Beta Subunit====== | ======Beta Subunit====== | ||
The beta subunit is the membrane spanning and intracellular portion of the insulin receptor. This domain is composed of three Fibronectin domains (FN III-1,-2,-3) and the tyrosine kinase domain. The tyrosine kinase domain is the site of autophosphorylation upon activation of the receptor. | The beta subunit is the membrane spanning and intracellular portion of the insulin receptor. This domain is composed of three Fibronectin domains (FN III-1,-2,-3) and the tyrosine kinase domain. The tyrosine kinase domain is the site of autophosphorylation upon activation of the receptor. | ||
| + | |||
===Conformation Change=== | ===Conformation Change=== | ||
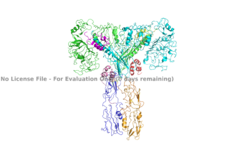
| - | The inactive form of the insulin receptor predominates in low-levels of circulating insulin, whereas the active conformation is seen when insulin binds to any of the 4 receptor sites. The inactive conformation resembles an <scene name='83/832953/Simple_inactivated_receptor/1'>inverted V</scene>, and the active conformation resembles a T (Figure 2). The image of the inverted V conformation shows only a protomer of the inactive alpha subunit because the entire inactive alpha subunit dimer has been unable to be photographed because the transition state has yet to be determined. Upon the binding of insulin to any of the four binding sites, the conformation change will begin, | + | The inactive form of the insulin receptor predominates in low-levels of circulating insulin, whereas the active conformation is seen when insulin binds to any of the 4 receptor sites. The inactive conformation resembles an <scene name='83/832953/Simple_inactivated_receptor/1'>inverted V</scene>, and the active conformation resembles a T (Figure 2). The image of the inverted V conformation shows only a protomer of the inactive alpha subunit because the entire inactive alpha subunit dimer has been unable to be photographed because the transition state has yet to be determined in full. In the V-shape, the FnIII-3 domains are separated by about 120A. At this distance, they cannot work together to autophosphorylate. Upon the binding of insulin to any of the four binding sites, the conformation change will begin and bring the FnIII-3 domains within 40A of each other, which is the T-state conformation. Cryo-EM results have displayed clear representations of FnIII-2 and FnIII-3 domains, but lack in high density results for the transmembrane domain and cannot truly model anything past the two fibronectin domains due to the lack of side chain density. It is suggested though, that the insulin receptor does extend its T-shape conformation through the cell membrane and into the cell. Therefore, it is expected that the intracellular kinase domains will be in close proximity when this conformation change occurs extracellularly, ultimately allowing for autophosphorylation. <ref> DOI 10.1038/s41467-018-06826-6</ref>. |
The insulin receptor can maximally bind four insulins to the active T-state at the four distinct insulin binding sites. Once insulin binds, the structural change from inactive to active state is stabilized by a tripartite interaction between insulin, L1, the C-terminal alpha helix, and the FNIII-1 domain. Studies have found that optimal insulin receptor activation requires the binding of multiple insulin ligands to two insulin binding sites. In (Figure 3) these two binding sites are colored in magenta and red. Binding of at least one insulin to the red binding site in (Figure 3) is required for the activation of the insulin receptor and the change in conformation to the active T state. <ref> DOI 10.7554/eLife.48630 </ref>. | The insulin receptor can maximally bind four insulins to the active T-state at the four distinct insulin binding sites. Once insulin binds, the structural change from inactive to active state is stabilized by a tripartite interaction between insulin, L1, the C-terminal alpha helix, and the FNIII-1 domain. Studies have found that optimal insulin receptor activation requires the binding of multiple insulin ligands to two insulin binding sites. In (Figure 3) these two binding sites are colored in magenta and red. Binding of at least one insulin to the red binding site in (Figure 3) is required for the activation of the insulin receptor and the change in conformation to the active T state. <ref> DOI 10.7554/eLife.48630 </ref>. | ||
Revision as of 02:54, 6 April 2020
Homo sapiens Insulin Receptor
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39. PMID:16278749
- ↑ Riddle MC. Treatment of diabetes with insulin. From art to science. West J Med. 1983 Jun;138(6):838-46. PMID:6351440
Student Contributors
- Harrison Smith
- Alyssa Ritter