Sandbox Reserved 1606
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
ABCG2 hinders cancer treatment by contributing to [https://en.wikipedia.org/wiki/Multiple_drug_resistance multidrug resistance] in tumor cells. ABCG2 exports xenbiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. The inhibition of ABCG2 would stop the transport of anti-cancer drugs out of cancer cells. Due to the potential the inhibition of ABCG2 to aid in cancer treatment, efforts have gone towards the development of specific inhibitors of ABCG2 and other ABC transporters. Inhibitors were derived from fungal toxin fumitremorgin C; however, many of those developed have neurotoxic effects.<ref name="Jackson"/> | ABCG2 hinders cancer treatment by contributing to [https://en.wikipedia.org/wiki/Multiple_drug_resistance multidrug resistance] in tumor cells. ABCG2 exports xenbiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. The inhibition of ABCG2 would stop the transport of anti-cancer drugs out of cancer cells. Due to the potential the inhibition of ABCG2 to aid in cancer treatment, efforts have gone towards the development of specific inhibitors of ABCG2 and other ABC transporters. Inhibitors were derived from fungal toxin fumitremorgin C; however, many of those developed have neurotoxic effects.<ref name="Jackson"/> | ||
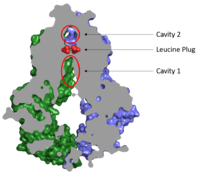
| + | <scene name='83/832932/Inhibitor_bound_cavity_1/1'>Inhibitors bind Cavity 1</scene> and act as competitive inhibitors against ABCG2 substrates. Depending on the size of the inhibitor, one or two molecules can bind to "wedge and clog" the transporter locking it in the inward-facing conformation. Inhibitors form many of the same binding interactions as substrates in Cavity 1. | ||
Revision as of 20:39, 6 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
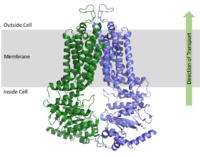
ABCG2 Multidrug Transporter
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ 3.0 3.1 Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. PMID:29643473 doi:http://dx.doi.org/10.1038/s41568-018-0005-8
- ↑ 4.0 4.1 4.2 Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
Student Contributors
Julia Pomeroy