Sandbox Reserved 1606
From Proteopedia
(Difference between revisions)
| Line 9: | Line 9: | ||
== Structural highlights == | == Structural highlights == | ||
| + | ===Overall Structure=== | ||
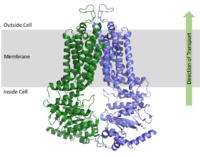
ABCG2 is a homodimer with each subunit containing two domains, the nucleotide binding domain <scene name='83/832932/Overall_structure_nbd_unbound/1'>(NBD)</scene> and the transmembrane domain <scene name='83/832932/Overall_structure_tmd_unbound/2'>(TMD)</scene>, which are fused together as a single peptide chain.<ref name="Taylor"/> The NBD is located inside of the cell and exposed to the cytosol while the TMD is embedded in the cell membrane and exposed to both the extracellular space and cytosol. [[Image:membrane domains.png|200 px|right|thumb|Figure 1. Domains of ABCG2 Multidrug transporter. The above protein is in the inward-facing conformation]] | ABCG2 is a homodimer with each subunit containing two domains, the nucleotide binding domain <scene name='83/832932/Overall_structure_nbd_unbound/1'>(NBD)</scene> and the transmembrane domain <scene name='83/832932/Overall_structure_tmd_unbound/2'>(TMD)</scene>, which are fused together as a single peptide chain.<ref name="Taylor"/> The NBD is located inside of the cell and exposed to the cytosol while the TMD is embedded in the cell membrane and exposed to both the extracellular space and cytosol. [[Image:membrane domains.png|200 px|right|thumb|Figure 1. Domains of ABCG2 Multidrug transporter. The above protein is in the inward-facing conformation]] | ||
===ATP Bound and Unbound Conformations=== | ===ATP Bound and Unbound Conformations=== | ||
| - | As an [https://en.wikipedia.org/wiki/ATP-binding_cassette_transporter ABC Transporter], ABCG2 exhibits ATPase activity in which the protein binds and hydrolyzes ATP. After the binding of a substrate, one molecule of <scene name='83/832932/Atp_bound_use2/1'>ATP binds each NBD</scene> causing a conformational change of the overall structure from an <scene name='83/832932/Overall_use_2/1'>inward-facing conformation</scene> | + | As an [https://en.wikipedia.org/wiki/ATP-binding_cassette_transporter ABC Transporter], ABCG2 exhibits ATPase activity in which the protein binds and hydrolyzes ATP. After the binding of a substrate, one molecule of <scene name='83/832932/Atp_bound_use2/1'>ATP binds each NBD</scene> causing a conformational change of the overall structure from an <scene name='83/832932/Overall_use_2/1'>inward-facing conformation</scene> to an <scene name='83/832932/Outward_facing_conformation/1'>outward-facing conformation</scene>. One molecule of ATP is hydrolyzed to transport substrates across the cell membrane while the second molecule of ATP is hydrolyzed to reset the transporter to its inward-facing conformation.<ref name="Robey"/> |
| - | When ATP binds, α-helices in | + | When ATP binds, α-helices in the NBD <scene name='83/832932/Atp_bound_nbd/1'>rotate</scene> approximately 35° relative to the <scene name='83/832932/Overall_structure_nbd_unbound/1'>inward-facing conformations</scene>. This shift in the NBD causes slight shifts of α-helices in the TMD; these helices are <scene name='83/832932/Atp_bound_use_tmd/1'>pushed toward each other</scene> relative to the <scene name='83/832932/Overall_structure_tmd_unbound/2'>inward-facing conformations</scene>. The overall shift from inward-facing to outward-facing promotes the transport of substrates through the transporter.<ref name="Manolaridis"/> |
===Cavities and Leucine Plug=== | ===Cavities and Leucine Plug=== | ||
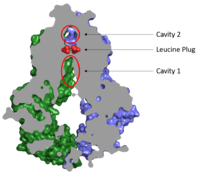
[[Image:Map_leucine_plug.png|200 px|right|thumb|Figure 2. Locations of Cavities 1 and 2 and the Leucine Plug in ABCG2. The above protein is in the inward-facing conformation]] | [[Image:Map_leucine_plug.png|200 px|right|thumb|Figure 2. Locations of Cavities 1 and 2 and the Leucine Plug in ABCG2. The above protein is in the inward-facing conformation]] | ||
| - | Substrates are transported through ABCG2 via two cavities separated by a leucine plug. <scene name='83/832932/Cavity_1_zoom_out/1'>Cavity 1</scene> acts as | + | Substrates are transported through ABCG2 via two cavities separated by a leucine plug. <scene name='83/832932/Cavity_1_zoom_out/1'>Cavity 1</scene> acts as a multidrug binding pocket and is formed by helices at the interface of the subunits in the TMD. When ATP is not bound to the NBDs, Cavity 1 is <scene name='83/832932/Overall_structure_cavity_1hel/2'>open to the cytosol</scene> in order to recruit substrates for transport. Cavity 1 is <scene name='83/832932/Cavity_1_narrow_surface/1'>narrow</scene> and full of nonpolar, hydrophobic residues and, as a result, prefers nonpolar, hydrophobic substrates, particularly flat, polycyclic molecules.<ref name="Taylor"/> Substrates, such as estrone sulfate, <scene name='83/832932/Cavity_1_-_use2/2'>form hydrogen bonds and stacking interactions</scene> with residues from each subunit in Cavity 1. |
| - | After substrates bind in Cavity 1, ATP binds each NBD leading to the | + | After substrates bind in Cavity 1, ATP binds each NBD leading to the transporter shifting from inward-facing to outward-facing. The outward-facing conformation results in the <scene name='83/832932/Atp_bound_cavity_2/2'>collapse of Cavity 1</scene> in the TMD which closes Cavity 1 to the cytosol and forces the substrate to move forward to Cavity 2 as there is no longer room in Cavity 1 to accommodate substrates.<ref name="Manolaridis"/> <scene name='83/832932/Atp_bound_use_cav_2/1'>Cavity 2</scene>, which is occluded when in the inward-facing conformation, is now open to the extracellular space and able to release the substrate. Cavity 2 contains a less hydrophobic environment and, as a result, substrates are released due to hydrophobic mismatch.<ref name="Taylor"/> <scene name='83/832932/Atp_bound_use_el_disulfides/1'>Disulfide bonds</scene> in the external loop near the exit of Cavity 2 also help promote substrate release.<ref name="Manolaridis"/> Once Cavity 2 is empty, the protein can be converted back to the inward-facing conformation via hydrolysis of ATP. |
| - | Cavities 1 and 2 are separated by a <scene name='83/832932/Overall_structure_cavsleu/1'>leucine plug</scene> which likely acts as a substrate check-point during transport; removal of this plug has exhibited an increase in transport and a decrease in substrate specificity.<ref name="Manolaridis"/> After the substrate binds Cavity 1 and ATP molecules bind | + | Cavities 1 and 2 are separated by a <scene name='83/832932/Overall_structure_cavsleu/1'>leucine plug</scene> which likely acts as a substrate check-point during transport; removal of this plug has exhibited an increase in transport and a decrease in substrate specificity.<ref name="Manolaridis"/> After the substrate binds Cavity 1 and ATP molecules bind each NBD, the <scene name='83/832932/Atp_bound_cavsleu/1'>leucine plug opens</scene> to allow the substrate to enter Cavity 2. Once the substrate enters Cavity 2, the plug is able to reform and promote substrate release and conversion to the inward-facing conformation. |
==Disease== | ==Disease== | ||
| - | Dysfunctions in ABCG2 | + | Dysfunctions in ABCG2 are linked to hyperuricemia which can lead to gout, kidney disease, and hypertension, all of which are thought to be the result of impaired transport of uric acid. Additionally, the expression of ABCG2 correlates to poor prognosis and treatment outcome of certain cancers such as breast, ovarian, and lung.<ref name="Jackson"/> |
===Cancer=== | ===Cancer=== | ||
| Line 31: | Line 32: | ||
===ABCG2 Inhibitors=== | ===ABCG2 Inhibitors=== | ||
| - | <scene name='83/832932/Inhibitor_bound_cavity_1/1'>Inhibitors bind Cavity 1</scene>, acting as competitive inhibitors against ABCG2 substrates. Depending on the size of the inhibitor, one or two molecules are required to bind to the cavity and form <scene name='83/832932/Inhibitor_interactions_cavity1/1'>hydrogen bonds, van der Waals, and stacking interactions</scene> within the binding site. It's suggested that many inhibitors are too big to be transported via the leucine plug resulting in the "clogging" of the transporter. With inhibitors acting as wedges, ABCG2 is locked in the inward-facing conformation and unable to transport molecules out of the cell. | + | <scene name='83/832932/Inhibitor_bound_cavity_1/1'>Inhibitors bind Cavity 1</scene>, acting as competitive inhibitors against ABCG2 substrates. Depending on the size of the inhibitor, one or two molecules are required to bind to the cavity and form <scene name='83/832932/Inhibitor_interactions_cavity1/1'>hydrogen bonds, van der Waals, and stacking interactions</scene> within the binding site.<ref name="Jackson"/> It's suggested that many inhibitors are too big to be transported via the leucine plug resulting in the "clogging" of the transporter. With inhibitors acting as wedges, ABCG2 is locked in the inward-facing conformation and unable to transport molecules out of the cell.<ref name="Manolaridis"/> |
</StructureSection> | </StructureSection> | ||
Revision as of 22:28, 6 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
ABCG2 Multidrug Transporter
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ 3.0 3.1 Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. PMID:29643473 doi:http://dx.doi.org/10.1038/s41568-018-0005-8
- ↑ 4.0 4.1 4.2 4.3 Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
Student Contributors
Julia Pomeroy