Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
===Substrate Structure=== | ===Substrate Structure=== | ||
[[Image:App.png|250 px|right|thumb|Structure of the substrate APP]] | [[Image:App.png|250 px|right|thumb|Structure of the substrate APP]] | ||
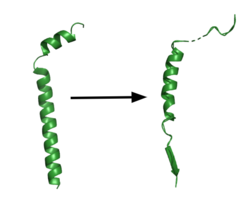
| - | Though gamma secretase has multiple substrates, the substrate of main concern is called [https://en.wikipedia.org/wiki/Amyloid_precursor_protein Amyloid Precursor Protein (APP).] APP is composed of an N-terminal loop, a transmembrane (TM) helix, and a C-terminal β-strand. The uses lateral diffusion as a mechanism of entry into the enzyme, and once in place, the TM helix is anchored by hydrogen bonds. In order to differentiate substrates, the β-strand is often the main point of identification for the enzyme. After this, the helix undergoes unwinding and the process of catalysis can begin. | + | Though gamma secretase has multiple substrates, the substrate of main concern is called [https://en.wikipedia.org/wiki/Amyloid_precursor_protein Amyloid Precursor Protein (APP).] APP is composed of an N-terminal loop, a transmembrane (TM) helix, and a C-terminal β-strand. The uses lateral diffusion as a mechanism of entry into the enzyme, and once in place, the TM helix is anchored by hydrogen bonds. In order to differentiate substrates, the β-strand is often the main point of identification for the enzyme. After this, the helix undergoes unwinding and the process of catalysis can begin<ref name="Zhou">PMID:30630874</ref>. |
===Lid Complex=== | ===Lid Complex=== | ||
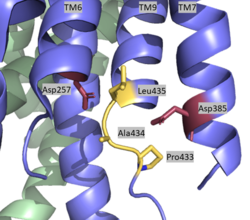
| - | The <scene name='83/832945/Lidremake/1'>lid complex</scene> is the first point of entry and recognition for the substrate. This lid is located within the NCT subunit between Asn55 and Asn435. This lobe is divided into two separate subunits, and Phe287 from large lobe acts as pivot between them. This <scene name='83/832945/Pivot/1'>Phe is further surrounded by Phe103, Leu171, Phe176, and Ile180</scene> of the small subunit, and these residues compose a greasy pocket for that provides an environment for easy movement. The lid consists of 5 aromatic residues which are highly involved with stabilizing the closed conformation. In particular, this conformation is stabilized by <scene name='83/832945/Trp164scene/1'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic pocket is revealed. This pocket contains <scene name='83/832945/Gluandtyr_remake/1'>Glu333 and Tyr337 surrounded by several charged residues</scene>, and is involved with further substrate binding and recognition once the lid is removed. | + | The <scene name='83/832945/Lidremake/1'>lid complex</scene> is the first point of entry and recognition for the substrate. This lid is located within the NCT subunit between Asn55 and Asn435. This lobe is divided into two separate subunits, and Phe287 from large lobe acts as pivot between them. This <scene name='83/832945/Pivot/1'>Phe is further surrounded by Phe103, Leu171, Phe176, and Ile180</scene> of the small subunit, and these residues compose a greasy pocket for that provides an environment for easy movement. The lid consists of 5 aromatic residues which are highly involved with stabilizing the closed conformation. In particular, this conformation is stabilized by <scene name='83/832945/Trp164scene/1'>Trp164, which interacts with Pro424, Phe448, and the aliphatic side chain of Gln420</scene>. Once the substrate binds and the lid is opened, a charged, hydrophilic pocket is revealed. This pocket contains <scene name='83/832945/Gluandtyr_remake/1'>Glu333 and Tyr337 surrounded by several charged residues</scene>, and is involved with further substrate binding and recognition once the lid is removed<ref name="Bai">PMID:26280335</ref> . |
| Line 34: | Line 34: | ||
==Relevance== | ==Relevance== | ||
| - | Gamma secretase has been determined to be highly involved with diseases such as Alzheimer's disease (AD). In this, beta-amyloid build up leads to [https://en.wikipedia.org/wiki/Amyloid amyloid]plaques in brain. These plaques then go on to cause severe neural dysfunction over time. Inhibition of gamma secretase could be potential AD treatment, but as stated earlier, this is a hard model to accomplish as gamma secretase is relevant with several different substrates. Complete inhibition would cause other severe problems beyond that of AD, making treatment more difficult than what meets the eye. However, what is known is that there are many different regions that give rise to gamma secretase malfunction when they are mutated. Over 200 of these mutations have been linked to causing AD. In particular, these mutations target so called "hot spots" on the enzyme and heavily impact the interface between PS1 and APP, affecting the integrity of catalysis and ultimately creating the plaques that impair neural function. Currently, in order to combat this complex situation, differences in binding between different substrates are being used to create drugs that selectively inhibit APP binding with gamma secretase, and possibly create a more ideal target for AD treatment. | + | Gamma secretase has been determined to be highly involved with diseases such as Alzheimer's disease (AD). In this, beta-amyloid build up leads to [https://en.wikipedia.org/wiki/Amyloid amyloid]plaques in brain. These plaques then go on to cause severe neural dysfunction over time. Inhibition of gamma secretase could be potential AD treatment, but as stated earlier, this is a hard model to accomplish as gamma secretase is relevant with several different substrates. Complete inhibition would cause other severe problems beyond that of AD, making treatment more difficult than what meets the eye. However, what is known is that there are many different regions that give rise to gamma secretase malfunction when they are mutated. Over 200 of these mutations have been linked to causing AD. In particular, these mutations target so called "hot spots" on the enzyme and heavily impact the interface between PS1 and APP, affecting the integrity of catalysis and ultimately creating the plaques that impair neural function. Currently, in order to combat this complex situation, differences in binding between different substrates are being used to create drugs that selectively inhibit APP binding with gamma secretase, and possibly create a more ideal target for AD treatment<ref name="Zhou">PMID:30630874</ref>. |
Revision as of 04:43, 7 April 2020
Gamma Secretase
| |||||||||||
References
- ↑ Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 2.0 2.1 2.2 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
Student Contributors
Layla Wisser
Daniel Mulawa