We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madison Summers/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 10: | Line 10: | ||
== Structural highlights and mechanism == | == Structural highlights and mechanism == | ||
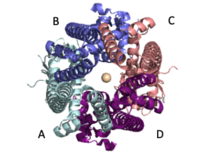
| - | The MCU is a dimer of dimers, described as tetrameric truncated pyramid. The uniporter has only a single strong binding site located in the selectivity pore with specificity for [https://en.wikipedia.org/wiki/Calcium_signaling Calcium], near the surface of the inner mitochondrial membrane. <ref name="Fan C"> DOI: 10.1038/s41586-018-0330-9</ref> Activity of the uniporter is dependent on membrane potential and calcium concentration. Calcium from the cytoplasm enters the mitochondrial inner membrane space through the mitochondrial membrane and is passed to the mitochondrial matrix via the MCU. [[Image:structure.png|300 px|right|thumb|Figure 2: structure of mitochondrial calcium uniporter colored by functional domain. The transmembrane domain is highlighted salmon, the matrix in light cyan, coiled coil in dark violet, and the N-Terminal Domain in slate blue. Each domain has a different functional role [https://www.rcsb.org/structure/6DT0 6DT0]] | + | The MCU is a dimer of dimers, described as tetrameric truncated pyramid. The uniporter has only a single strong binding site located in the selectivity pore with specificity for [https://en.wikipedia.org/wiki/Calcium_signaling Calcium], near the surface of the inner mitochondrial membrane. <ref name="Fan C"> DOI: 10.1038/s41586-018-0330-9</ref> Activity of the uniporter is dependent on membrane potential and calcium concentration. Calcium from the cytoplasm enters the mitochondrial inner membrane space through the mitochondrial membrane and is passed to the mitochondrial matrix via the MCU. [[Image:structure.png|300 px|right|thumb|Figure 2: structure of mitochondrial calcium uniporter colored by functional domain. The transmembrane domain is highlighted salmon, the matrix in light cyan, coiled coil in dark violet, and the N-Terminal Domain in slate blue. Each domain has a different functional role [https://www.rcsb.org/structure/6DT0 6DT0] ] |
===Transmembrane Domain=== | ===Transmembrane Domain=== | ||
The <scene name='83/837230/Transmembrane_domain/3'>transmembrane domain</scene> is on the [https://en.wikipedia.org/wiki/Mitochondrion#Structure inner mitochondrial membrane] open to the inner membrane space. The small pore, highly specific for calcium binding is located in <scene name='83/837230/Tm2/1'>transmembrane 2</scene> (TM2) while <scene name='83/837230/Transmembrane_1/2'>transmembrane 1</scene> (TM1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. It is important that the selectivity pore is small, allowing only a dehydrated calcium molecule to interact with the 5 ampier wide glutamate ring. The negative charge of the glutamates carboxyl group attracts the positively charged Calcium ion. Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12 ampier wide pore allowing high conductivity. <ref name="Fan C" /> The wider opening allows calcium to rehydrate once they pass the selectivity pore. The domain swapping of TM1 of one subunit with the TM2 of the neighboring subunits allows for a tight packing in the transmembrane connectivity providing flexibility to the uniporter. | The <scene name='83/837230/Transmembrane_domain/3'>transmembrane domain</scene> is on the [https://en.wikipedia.org/wiki/Mitochondrion#Structure inner mitochondrial membrane] open to the inner membrane space. The small pore, highly specific for calcium binding is located in <scene name='83/837230/Tm2/1'>transmembrane 2</scene> (TM2) while <scene name='83/837230/Transmembrane_1/2'>transmembrane 1</scene> (TM1) surrounds the pore. The transmembrane domain exhibits four fold rotational symmetry. It is important that the selectivity pore is small, allowing only a dehydrated calcium molecule to interact with the 5 ampier wide glutamate ring. The negative charge of the glutamates carboxyl group attracts the positively charged Calcium ion. Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12 ampier wide pore allowing high conductivity. <ref name="Fan C" /> The wider opening allows calcium to rehydrate once they pass the selectivity pore. The domain swapping of TM1 of one subunit with the TM2 of the neighboring subunits allows for a tight packing in the transmembrane connectivity providing flexibility to the uniporter. | ||
Revision as of 19:38, 10 April 2020
Mitochondrial Calcium Uniporter
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 2.0 2.1 2.2 2.3 2.4 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9
- ↑ Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018 Jun 28. pii: science.aar4056. doi: 10.1126/science.aar4056. PMID:29954988 doi:http://dx.doi.org/10.1126/science.aar4056