We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1627
From Proteopedia
(Difference between revisions)
| Line 27: | Line 27: | ||
===Binding interactions=== | ===Binding interactions=== | ||
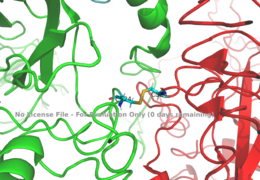
[[Image:Binding site with AA labeled.png|thumb|right|270px|Figure 3: Subunit interactions between the insulin receptor CT-alpha helix (light blue) and insulin (magenta) in one of the binding sites. [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | [[Image:Binding site with AA labeled.png|thumb|right|270px|Figure 3: Subunit interactions between the insulin receptor CT-alpha helix (light blue) and insulin (magenta) in one of the binding sites. [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | ||
| - | A tripartite interaction occurs between the alpha-CT chain and the FnIII-1 domain region during the conformational change. This interaction involves the following residues:<scene name='83/832953/Alpha_ct_and_fniii-1/4'>ASP496, ARG498, and ASP499 on the FnIII-1 domain</scene> and the <scene name='83/832953/Alpha_ct_and_fniii-1/5'>LYS703, GLU706, and ASP707 on the alpha-CT domain</scene>. This duo then interacts with the leucine rich region, L1, that exists on the opposing protomer of the dimer. | + | A tripartite interaction occurs between the alpha-CT chain and the FnIII-1 domain region during the conformational change. This interaction involves the following residues:<scene name='83/832953/Alpha_ct_and_fniii-1/4'>ASP496, ARG498, and ASP499 on the FnIII-1 domain</scene> and the <scene name='83/832953/Alpha_ct_and_fniii-1/5'>LYS703, GLU706, and ASP707 on the alpha-CT domain</scene>. This duo then interacts with the leucine rich region, L1, that exists on the opposing protomer of the dimer. The tripartite interaction between the alpha-CT chain and FnIII-1 domain on one dimer and the L1 region on the other dimer is important because it allows for a strong and stable interaction between two dimers of the insulin receptor that maintains the T-shape activation state for the rest of the downstream signaling to occur. |
| + | |||
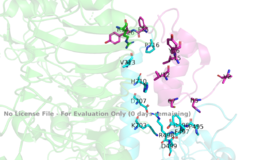
[[Image:4 sites highlighted.png|thumb|right|260px|Figure 4: The presence of all four potential binding sides on the active insulin receptor: sites 1 and 1' (green) and sites 2, and 2'(red). [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | [[Image:4 sites highlighted.png|thumb|right|260px|Figure 4: The presence of all four potential binding sides on the active insulin receptor: sites 1 and 1' (green) and sites 2, and 2'(red). [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | ||
For insulin binding, it is generally more common for only one or two insulin molecules to bind to the receptor due to the occurrence of negative [http://en.wikipedia.org/wiki/Ligand_(biochemistry)#Receptor.2Fligand_binding_affinity affinity] at the binding site. The location of the second two binding sites are on the back side of the Beta subunits and lack favorable surface area. The binding of ligands to two insulin binding sites necessitates optimal insulin receptor activation. Binding of at least one insulin is required for the activation of the insulin receptor and the change in conformation to the active T state. <ref> DOI 10.7554/eLife.48630 </ref>. | For insulin binding, it is generally more common for only one or two insulin molecules to bind to the receptor due to the occurrence of negative [http://en.wikipedia.org/wiki/Ligand_(biochemistry)#Receptor.2Fligand_binding_affinity affinity] at the binding site. The location of the second two binding sites are on the back side of the Beta subunits and lack favorable surface area. The binding of ligands to two insulin binding sites necessitates optimal insulin receptor activation. Binding of at least one insulin is required for the activation of the insulin receptor and the change in conformation to the active T state. <ref> DOI 10.7554/eLife.48630 </ref>. | ||
Revision as of 22:17, 16 April 2020
Homo sapiens Insulin Receptor
| |||||||||||
References
- ↑ 1.0 1.1 De Meyts P. The Insulin Receptor and Its Signal Transduction Network PMID:27512793
- ↑ 2.0 2.1 2.2 Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ 3.0 3.1 3.2 Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1). pii: 6/1/a009191. doi:, 10.1101/cshperspect.a009191. PMID:24384568 doi:http://dx.doi.org/10.1101/cshperspect.a009191
- ↑ Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39. PMID:16278749
- ↑ Riddle MC. Treatment of diabetes with insulin. From art to science. West J Med. 1983 Jun;138(6):838-46. PMID:6351440
Student Contributors
- Harrison Smith
- Alyssa Ritter