We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1627
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
====Alpha Subunits==== | ====Alpha Subunits==== | ||
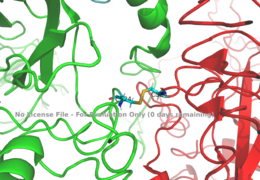
[[Image:Disulfide bridge between alphas.png|thumb|right|260px|Figure 1: Disulfide bridge (yellow) made of two cysteine residues (blue) that provides a linkage and stability to the two alpha subunits. [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | [[Image:Disulfide bridge between alphas.png|thumb|right|260px|Figure 1: Disulfide bridge (yellow) made of two cysteine residues (blue) that provides a linkage and stability to the two alpha subunits. [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | ||
| - | The alpha subunits make up the extracellular domain ([http://en.wikipedia.org/wiki/Ectodomain ectodomain]) of the insulin receptor and are the sites of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a C-terminal alpha helix. The alpha and beta subunits are held together by a [http://en.wikipedia.org/wiki/Disulfide disulfide bond] at residue C524 of one alpha subunit, and C524 of the other subunit (Figure1). The actual site of insulin binding occurs at the <scene name='83/832953/Alpha_c_helix/3'>α-CT chain</scene> of one of the sites discussed next and is stabilized by the L1 and L2 domains. Two types of insulin binding sites are present in the alpha subunits, Sites 1 and 1' and then Sites 2 and 2'. Due to structural differences in these binding sites, the first two sites, 1 and 1', have much higher affinity than that of sites 2 and 2'. The sites are in pairs because of the heterodimeric nature of the receptor. Each time an insulin ligand binds to sites 1 and 1', it comes in contact with the L1 domain of one protomer and the alpha-CT chain and FnIII-1 loop of another protomer, which is also known as "cross linking". Insulin can also bind at sites 2 and 2', but the location on the back of the beta sheet of the FnIII-1 domain and lower surface area decreases their binding occupancy. <ref name="Uchikawa"> DOI 10.7554/eLife.48630 </ref> | + | The alpha subunits make up the extracellular domain ([http://en.wikipedia.org/wiki/Ectodomain ectodomain]) of the insulin receptor and are the sites of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a C-terminal alpha helix. The alpha and beta subunits are held together by a [http://en.wikipedia.org/wiki/Disulfide disulfide bond] at residue C524 of one alpha subunit, and C524 of the other subunit (Figure1). The actual site of insulin binding occurs at the <scene name='83/832953/Alpha_c_helix/3'>α-CT chain</scene> of one of the sites discussed next and is stabilized by the L1 and L2 domains. Two types of insulin binding sites are present in the alpha subunits, Sites 1 and 1' and then Sites 2 and 2'. Due to structural differences in these binding sites, the first two sites, 1 and 1', have much higher affinity than that of sites 2 and 2'. The sites are in pairs because of the heterodimeric nature of the receptor. Each time an insulin ligand binds to sites 1 and 1', it comes in contact with the L1 domain of one protomer and the alpha-CT chain and FnIII-1 loop of another protomer, which is also known as "cross linking". Insulin can also bind at sites 2 and 2', but the location on the back of the beta sheet of the FnIII-1 domain and lower surface area decreases their binding occupancy. <ref name="Uchikawa"> DOI 10.7554/eLife.48630 </ref> Cryo-EM has imaged insulin bound structures that displayed a T-shape conformation in the alpha subunits.<ref name="Uchikawa" /> |
===Beta Subunits=== | ===Beta Subunits=== | ||
| Line 23: | Line 23: | ||
===Conformation Change=== | ===Conformation Change=== | ||
| - | Structures of the inactive inverted V conformation only contains a single <scene name='83/832953/Inactive_insulin_receptor/3'>protomer of the extracellular alpha and beta subunits</scene> because the entire inactive alpha subunit dimer has been unable to be photographed because the transition state has yet to be determined in full. In the V-shape, the FnIII-3 domains are separated by about 120Å which keeps the tyrosine kinase domains separated. In the V-shape, autophosphorylation and downstream signaling cannot be initiated. Upon the binding of insulin to | + | Structures of the inactive inverted V conformation only contains a single <scene name='83/832953/Inactive_insulin_receptor/3'>protomer of the extracellular alpha and beta subunits</scene> because the entire inactive alpha subunit dimer has been unable to be photographed because the transition state has yet to be determined in full. In the V-shape, the FnIII-3 domains are separated by about 120Å which keeps the tyrosine kinase domains separated. In the V-shape, autophosphorylation and downstream signaling cannot be initiated. Upon the binding of insulin to binding sites 1, 1', and either 2 or 2', the conformation change will begin and bring the FnIII-3 domains within 40Å of each other to induce the T-state conformation. <ref> DOI 10.1038/s41467-018-06826-6</ref> <ref name="Uchikawa" /> The T shape conformation is well observed in the alpha subunit. It is horizontally composed of L1, CR (including the alpha-CT chain), and L2 domains and vertically composed of the FnIII-1, 2, and 3 domains. This structural transition will facilitate the autophosphorylation of the tyrosine kinase domain. |
===Binding interactions=== | ===Binding interactions=== | ||
| Line 30: | Line 30: | ||
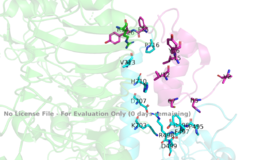
[[Image:4 sites highlighted.png|thumb|right|260px|Figure 4: The presence of all four potential binding sides on the active insulin receptor: sites 1 and 1' (green) and sites 2, and 2'(red). [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | [[Image:4 sites highlighted.png|thumb|right|260px|Figure 4: The presence of all four potential binding sides on the active insulin receptor: sites 1 and 1' (green) and sites 2, and 2'(red). [http://www.rcsb.org/structure/6sof PDB 6SOF]]] | ||
| - | For insulin binding, it is generally more common for only one or two insulin molecules to bind to the receptor due to the occurrence of negative [http://en.wikipedia.org/wiki/Ligand_(biochemistry)#Receptor.2Fligand_binding_affinity affinity] at the binding site. The location of the second two binding sites are on the back side of the Beta subunits and lack favorable surface area. The binding of | + | For insulin binding, it is generally more common for only one or two insulin molecules to bind to the receptor due to the occurrence of negative [http://en.wikipedia.org/wiki/Ligand_(biochemistry)#Receptor.2Fligand_binding_affinity affinity] at the binding site. The location of the second two binding sites are on the back side of the Beta subunits and lack favorable surface area. The binding of insulin to the binding sites 1 and 1', as well as one insulin to either binding site 2 or 2', is required for the activation of the insulin receptor and the change in conformation to the active T state. <ref> DOI 10.7554/eLife.48630 </ref>. |
Although interactions at all four binding sites are highly hydrophobic, ligand binding interactions are different at <scene name='83/832953/Take_14/1'>sites 1 and 1'</scene> and <scene name='83/832953/Sites_2_and_2_prime_location/6'>sites 2 and 2'</scene> (Figure 4). Sites 1 and 1' have two disulfide bond linkages, along with HIS B5 from insulin interacting with <scene name='83/832953/Sites_1_and_1_prime_location/7'>PRO495, PHE497, ARG498</scene> residues from the FnIII-1 domain. At sites 2 and 2' the FnIII-1 region has <scene name='83/832953/Sites_2_and_2_prime_location/8'>both basic residues-ARG479, LYS484, ARG488, ARG554- and hydrophobic residues- LEU486, LEU552, and PRO537</scene>- interacting with numerous insulin residues. | Although interactions at all four binding sites are highly hydrophobic, ligand binding interactions are different at <scene name='83/832953/Take_14/1'>sites 1 and 1'</scene> and <scene name='83/832953/Sites_2_and_2_prime_location/6'>sites 2 and 2'</scene> (Figure 4). Sites 1 and 1' have two disulfide bond linkages, along with HIS B5 from insulin interacting with <scene name='83/832953/Sites_1_and_1_prime_location/7'>PRO495, PHE497, ARG498</scene> residues from the FnIII-1 domain. At sites 2 and 2' the FnIII-1 region has <scene name='83/832953/Sites_2_and_2_prime_location/8'>both basic residues-ARG479, LYS484, ARG488, ARG554- and hydrophobic residues- LEU486, LEU552, and PRO537</scene>- interacting with numerous insulin residues. | ||
Revision as of 22:28, 16 April 2020
Homo sapiens Insulin Receptor
| |||||||||||
References
- ↑ 1.0 1.1 De Meyts P. The Insulin Receptor and Its Signal Transduction Network PMID:27512793
- ↑ 2.0 2.1 2.2 Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ 3.0 3.1 3.2 Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1). pii: 6/1/a009191. doi:, 10.1101/cshperspect.a009191. PMID:24384568 doi:http://dx.doi.org/10.1101/cshperspect.a009191
- ↑ Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39. PMID:16278749
- ↑ Riddle MC. Treatment of diabetes with insulin. From art to science. West J Med. 1983 Jun;138(6):838-46. PMID:6351440
Student Contributors
- Harrison Smith
- Alyssa Ritter