This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1626
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Overview == | == Overview == | ||
| - | |||
| - | [[Image:Electronegativity_MCU_4.jpg|300 px|right|thumb|'''Fig. 1''' MCU]] | ||
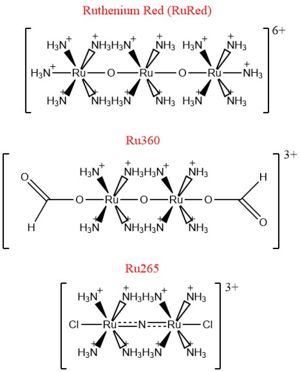
[[Image:Ruthenium_Inhibitors.jpg|300 px|right|thumb|'''Fig. 2''' Structure of Ruthenium Red]] | [[Image:Ruthenium_Inhibitors.jpg|300 px|right|thumb|'''Fig. 2''' Structure of Ruthenium Red]] | ||
| Line 26: | Line 24: | ||
===Selectivity Filter=== | ===Selectivity Filter=== | ||
| + | |||
| + | [[Image:Electronegativity_MCU_4.jpg|300 px|right|thumb|'''Fig. 1''' MCU]] | ||
The selectivity filter of the MCU is composed by many acidic amino acids near the narrow mouth of the channel which leads to high affinity for calcium ([https://en.wikipedia.org/wiki/Dissociation_constant dissociation constant] of less than 2nM).<ref name="Baradaran"/> The arrangement of the highly conserved WDXXEP [https://en.wikipedia.org/wiki/Sequence_motif motif] in the TM2 helices form a ring in the pore to which calcium ions are attracted.<ref name="Baradaran"/> The structure in the animation is the MCU of [http://www.mycobank.org/BioloMICS.aspx?Table=Mycobank&Rec=511257 ''Cyphellophora europaea''] so every amino acid named here specifically is that of ''C. europaea'', but most of these residues are highly conserved across all species, though residue number may change. Though not part of the WDXXEP motif, Asp221 is present at the mouth of the MCU and serves to congregate positively charged calcium ions at the entrance of the channel.<ref name="Baradaran"/> The WDXXEP motif consists of Trp224 at the N-terminal end, Asp225, Glu228, and Pro229.<ref name="Baradaran"/> Trp224 and Pro229 pack against each other and are oriented towards the pore, but only serve to stabilize Asp225 and Glu228, not interact with calcium ions.<ref name="Baradaran"/><ref name="Fan"/> The X residues (Val226 and Met227 in this case) face away from the pore and are exposed to the membrane.<ref name="Baradaran"/> The negatively charged side chains of Asp225 and Glu228 point towards the pore and form rings of radius 2.5Å and 1Å, respectively.<ref name="Baradaran"/> It's a combination of these radii and charges that account for the selectivity of the MCU. For example, potassium has an [https://en.wikipedia.org/wiki/Ionic_radius ionic radius] of 1.38Å which is much larger than the 1.00Å ionic radius of calcium.<ref name="Baradaran"/> Additionally, even though sodium ions have a similar ionic radius, the +2 charge on calcium is better matched to coordination with the glutamate residues.<ref name="Baradaran"/> | The selectivity filter of the MCU is composed by many acidic amino acids near the narrow mouth of the channel which leads to high affinity for calcium ([https://en.wikipedia.org/wiki/Dissociation_constant dissociation constant] of less than 2nM).<ref name="Baradaran"/> The arrangement of the highly conserved WDXXEP [https://en.wikipedia.org/wiki/Sequence_motif motif] in the TM2 helices form a ring in the pore to which calcium ions are attracted.<ref name="Baradaran"/> The structure in the animation is the MCU of [http://www.mycobank.org/BioloMICS.aspx?Table=Mycobank&Rec=511257 ''Cyphellophora europaea''] so every amino acid named here specifically is that of ''C. europaea'', but most of these residues are highly conserved across all species, though residue number may change. Though not part of the WDXXEP motif, Asp221 is present at the mouth of the MCU and serves to congregate positively charged calcium ions at the entrance of the channel.<ref name="Baradaran"/> The WDXXEP motif consists of Trp224 at the N-terminal end, Asp225, Glu228, and Pro229.<ref name="Baradaran"/> Trp224 and Pro229 pack against each other and are oriented towards the pore, but only serve to stabilize Asp225 and Glu228, not interact with calcium ions.<ref name="Baradaran"/><ref name="Fan"/> The X residues (Val226 and Met227 in this case) face away from the pore and are exposed to the membrane.<ref name="Baradaran"/> The negatively charged side chains of Asp225 and Glu228 point towards the pore and form rings of radius 2.5Å and 1Å, respectively.<ref name="Baradaran"/> It's a combination of these radii and charges that account for the selectivity of the MCU. For example, potassium has an [https://en.wikipedia.org/wiki/Ionic_radius ionic radius] of 1.38Å which is much larger than the 1.00Å ionic radius of calcium.<ref name="Baradaran"/> Additionally, even though sodium ions have a similar ionic radius, the +2 charge on calcium is better matched to coordination with the glutamate residues.<ref name="Baradaran"/> | ||
Revision as of 03:24, 18 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
Mitochondrial Calcium Uniporter (MCU) Complex
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0331-8. doi:, 10.1038/s41586-018-0331-8. PMID:29995857 doi:http://dx.doi.org/10.1038/s41586-018-0331-8
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Woods JJ, Wilson JJ. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr Opin Chem Biol. 2019 Dec 20;55:9-18. doi: 10.1016/j.cbpa.2019.11.006. PMID:31869674 doi:http://dx.doi.org/10.1016/j.cbpa.2019.11.006
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017 Sep 7;24(1):70. doi: 10.1186/s12929-017-0375-3. PMID:28882140 doi:http://dx.doi.org/10.1186/s12929-017-0375-3
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9
Student Contributors
Ryan Heumann
Rieser Wells