We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1625
From Proteopedia
(Difference between revisions)
| Line 25: | Line 25: | ||
Interestingly, the O-channel does not exist in the cytochrome ''bd'' oxidase of [https://www.rcsb.org/structure/5DOQ ''Geobacillus thermodenitrificans'']; instead, oxygen binds directly to the active site<ref name="Safarian2">PMID: 27126043</ref>. The <scene name='83/832931/Cyds/1'>CydS</scene> subunit found in E. coli blocks this alternate oxygen entry site, which allows oxygen to travel through the O-channel<ref name="Safarian">PMID:31604309</ref><ref name="Alexander">PMID:31723136</ref>. The presence of an o-channel affects oxidase activity, as the ''E. coli'' oxidase acts as a "true" oxidase, while the ''G. thermodenitrificans'' bd oxidase contributes more to detoxification<ref name="Alexander">PMID:31723136</ref>. | Interestingly, the O-channel does not exist in the cytochrome ''bd'' oxidase of [https://www.rcsb.org/structure/5DOQ ''Geobacillus thermodenitrificans'']; instead, oxygen binds directly to the active site<ref name="Safarian2">PMID: 27126043</ref>. The <scene name='83/832931/Cyds/1'>CydS</scene> subunit found in E. coli blocks this alternate oxygen entry site, which allows oxygen to travel through the O-channel<ref name="Safarian">PMID:31604309</ref><ref name="Alexander">PMID:31723136</ref>. The presence of an o-channel affects oxidase activity, as the ''E. coli'' oxidase acts as a "true" oxidase, while the ''G. thermodenitrificans'' bd oxidase contributes more to detoxification<ref name="Alexander">PMID:31723136</ref>. | ||
=== Hemes === | === Hemes === | ||
| - | Three <scene name='83/832931/Heme/6'>hemes</scene> are present in the CydA subunit. These three hemes form a triangle to maximize subunit stability<ref name="Safarian">PMID:31604309</ref><ref name="Alexander">PMID:31723136</ref><ref name="Safarian2">PMID:27126043</ref>, which is an evolutionary conserved feature across bd oxidases<ref name="Safarian">PMID:31604309</ref>. Heme b<sub>558</sub> acts as the primary electron acceptor by catalyzing the [https://en.wikipedia.org/wiki/Hydroquinone#Redox oxidation of quinol]<ref name="Alexander">PMID:31723136</ref>. Conserved <scene name='83/832931/Met393/1'>His186 and Met393</scene> help to stabilize heme b558<ref name="Alexander">PMID:31723136</ref>. Heme b<sub>558</sub> transfers the electrons to heme b595, which transfers them to the active site heme d<ref name= "Safarian">PMID:31604309</ref>. | + | Three <scene name='83/832931/Heme/6'>hemes</scene> are present in the CydA subunit. These three hemes form a triangle to maximize subunit stability<ref name="Safarian">PMID:31604309</ref><ref name="Alexander">PMID:31723136</ref><ref name="Safarian2">PMID:27126043</ref>, which is an evolutionary conserved feature across bd oxidases<ref name="Safarian">PMID:31604309</ref>. Heme b<sub>558</sub> acts as the primary electron acceptor by catalyzing the [https://en.wikipedia.org/wiki/Hydroquinone#Redox oxidation of quinol]<ref name="Alexander">PMID:31723136</ref>. Conserved <scene name='83/832931/Met393/1'>His186 and Met393</scene> help to stabilize heme b558<ref name="Alexander">PMID:31723136</ref>. Heme b<sub>558</sub> transfers the electrons to heme b595, which transfers them to the active site heme d<ref name= "Safarian">PMID:31604309</ref>. Multiple residues help stabilzie this electron trasnfer including a conserved <scene name='83/832931/Trp441/6'>Trp441</scene> that assists heme b<sub>595</sub> in transferring electrons to heme d<ref name="Safarian2">PMID:27126043</ref>. A conserved <scene name='83/832931/Hemeb595/2'>Glu445</scene> is also essential for charge stabilization of heme b<sub>595</sub><ref name="Alexander">PMID:31723136</ref>, while <scene name='83/832931/Hemeh19/3'>His19</scene> stabilizes heme d<ref name="Safarian2">PMID:27126043</ref>. As heme d collects the electrons from heme b<sub>595</sub>, <scene name='83/832931/Heme_d/3'>Glu99</scene> in the O-channel facilities the binding of oxygen to heme d, and <scene name='83/832931/Heme_d/3'>Ser108, Glu107, and Ser140</scene> in the h-channel facilitate proton transfer to heme d<ref name="Safarian">PMID:31604309</ref>. Similar to the three hemes, the <scene name='83/832931/Uq8/3'>ubiquinone-8</scene> (UQ-8) molecule found in CydB mimics the triangular formation to stabilize the subunit<ref name="Safarian">PMID:31604309</ref>. |
===Mechanism=== | ===Mechanism=== | ||
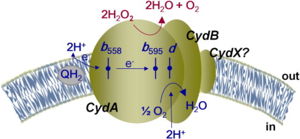
Quinol is used as the initial electron donor and heme b<sub>558</sub> is the initial electron acceptor. <scene name='83/832931/Heme/6'>Heme b<sub>558</sub></scene> transfers the electrons to <scene name='83/832931/Heme/6'>heme b<sub>595</sub></scene>, which transfers the electrons to <scene name='83/832931/Heme/6'>heme d</scene>. Concurrently, the <scene name='83/832931/Overall_h_channel/1'>H-channel</scene> will collect protons and <scene name='83/832931/O_channel_overall/2'>o-channel</scene> will collect oxygen atoms that will flow to heme d (Fig. 3). With electrons, oxygen, and protons available, heme d can successfully reduce dioxygen to water (Fig. 4). [[Image:mech4.png|500 px|center|thumb|''Figure 4''. Summarized mechanism of cytochrome bd-oxidase in ''E. coli''. Electrons are passed from quinol to heme b<sub>558</sub> to heme b<sub>595</sub> to heme d. Protons and oxygen atoms flow into the H-channel and O-channel to heme d. Heme d catalzyes the reduction of oxygen to water.]] | Quinol is used as the initial electron donor and heme b<sub>558</sub> is the initial electron acceptor. <scene name='83/832931/Heme/6'>Heme b<sub>558</sub></scene> transfers the electrons to <scene name='83/832931/Heme/6'>heme b<sub>595</sub></scene>, which transfers the electrons to <scene name='83/832931/Heme/6'>heme d</scene>. Concurrently, the <scene name='83/832931/Overall_h_channel/1'>H-channel</scene> will collect protons and <scene name='83/832931/O_channel_overall/2'>o-channel</scene> will collect oxygen atoms that will flow to heme d (Fig. 3). With electrons, oxygen, and protons available, heme d can successfully reduce dioxygen to water (Fig. 4). [[Image:mech4.png|500 px|center|thumb|''Figure 4''. Summarized mechanism of cytochrome bd-oxidase in ''E. coli''. Electrons are passed from quinol to heme b<sub>558</sub> to heme b<sub>595</sub> to heme d. Protons and oxygen atoms flow into the H-channel and O-channel to heme d. Heme d catalzyes the reduction of oxygen to water.]] | ||
== Relevance == | == Relevance == | ||
| - | The cytochrome ''bd'' oxidase is essential for bacteria to thrive in the human body by enhancing bacterial growth and colonization. Any alteration of the bd oxdiase Cyd subunits will most likely produce a nonfunctional mutant cytochrome ''bd'' oxidase<ref name="Moosa">PMID: 28760899</ref>, which inhibits bacterial growth. If ''E. coli'' were missing or possessed ineffective CydA and B subunits, bacterial growth ceased.<ref name="Hughes">PMID: 28182951</ref>. With [https://en.wikipedia.org/wiki/Colitis colitis], ''E. coli'' mutants that were missing CydAB colonized poorly in comparison to the wild type levels of colonization<ref name="Hughes">PMID: 28182951</ref>. The cytochrome ''bd'' oxidase is the main component in nitric oxide (NO) tolerance in bacteria, which is released by neutrophils and macrophages when the host is infected<ref name="Shepherd">PMID: 27767067</ref>. ''E. coli'' growth seen in urinary tract infections is mainly due to the NO resistant bd oxidase. Without the CydA and CydB subunits, bacteria could not colonize in high NO conditions<ref name="Shepherd">PMID: 27767067</ref>. Cytochrome ''bd'' oxidases are essential in other pathogenic bacteria such as [https://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''M. tuberculosis'']. Deletion of the CydA and CydB subunits dramatically decreased the growth of ''M. tb'' compared to the wild type when exposed to imidazo[1,2-α]pyridine, a known inhibitor of respiratory enzymes<ref name="Arora">PMID:25155596</ref>. Upregulation of the cytochrome ''bd'' oxidase Cyd genes resulted in a mutant strain of ''M. tb'' that was resistant to imidazo[1,2-α]pyridine<ref name="Arora">PMID:25155596</ref>. | + | The cytochrome ''bd'' oxidase is essential for bacteria to thrive in the human body by enhancing bacterial growth and colonization. Any alteration of the ''bd'' oxdiase Cyd subunits will most likely produce a nonfunctional mutant cytochrome ''bd'' oxidase<ref name="Moosa">PMID: 28760899</ref>, which inhibits bacterial growth. If ''E. coli'' were missing or possessed ineffective CydA and B subunits, bacterial growth ceased.<ref name="Hughes">PMID: 28182951</ref>. With [https://en.wikipedia.org/wiki/Colitis colitis], ''E. coli'' mutants that were missing CydAB colonized poorly in comparison to the wild type levels of colonization<ref name="Hughes">PMID: 28182951</ref>. The cytochrome ''bd'' oxidase is the main component in nitric oxide (NO) tolerance in bacteria, which is released by neutrophils and macrophages when the host is infected<ref name="Shepherd">PMID: 27767067</ref>. ''E. coli'' growth seen in urinary tract infections is mainly due to the NO resistant bd oxidase. Without the CydA and CydB subunits, bacteria could not colonize in high NO conditions<ref name="Shepherd">PMID: 27767067</ref>. Cytochrome ''bd'' oxidases are essential in other pathogenic bacteria such as [https://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''M. tuberculosis'']. Deletion of the CydA and CydB subunits dramatically decreased the growth of ''M. tb'' compared to the wild type when exposed to imidazo[1,2-α]pyridine, a known inhibitor of respiratory enzymes<ref name="Arora">PMID:25155596</ref>. Upregulation of the cytochrome ''bd'' oxidase Cyd genes resulted in a mutant strain of ''M. tb'' that was resistant to imidazo[1,2-α]pyridine<ref name="Arora">PMID:25155596</ref>. |
Since cytochrome ''bd'' oxidases are only found in prokaryotes and are required for pathogenic bacterial infections, inhibitors that target cytochrome ''bd'' oxidase are promising antibacterial agents. Compounds that target heme b<sub>558</sub><ref name="Harikishore">PMID: 31939065</ref>, create unusable forms of oxygen<ref name="Galván">PMID: 30790617</ref>, and target the o-channel <ref name="Lu">PMID: 26015371 </ref> have shown potential in halting bacterial growth. | Since cytochrome ''bd'' oxidases are only found in prokaryotes and are required for pathogenic bacterial infections, inhibitors that target cytochrome ''bd'' oxidase are promising antibacterial agents. Compounds that target heme b<sub>558</sub><ref name="Harikishore">PMID: 31939065</ref>, create unusable forms of oxygen<ref name="Galván">PMID: 30790617</ref>, and target the o-channel <ref name="Lu">PMID: 26015371 </ref> have shown potential in halting bacterial growth. | ||
Revision as of 00:01, 20 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
Cytochrome bd-1 oxidase in Escherichia coli
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 Safarian S, Rajendran C, Muller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 2016 Apr 29;352(6285):583-6. doi: 10.1126/science.aaf2477. PMID:27126043 doi:http://dx.doi.org/10.1126/science.aaf2477
- ↑ 2.0 2.1 Harikishore A, Chong SSM, Ragunathan P, Bates RW, Gruber G. Targeting the menaquinol binding loop of mycobacterial cytochrome bd oxidase. Mol Divers. 2020 Jan 14. pii: 10.1007/s11030-020-10034-0. doi:, 10.1007/s11030-020-10034-0. PMID:31939065 doi:http://dx.doi.org/10.1007/s11030-020-10034-0
- ↑ Boot M, Jim KK, Liu T, Commandeur S, Lu P, Verboom T, Lill H, Bitter W, Bald D. A fluorescence-based reporter for monitoring expression of mycobacterial cytochrome bd in response to antibacterials and during infection. Sci Rep. 2017 Sep 6;7(1):10665. doi: 10.1038/s41598-017-10944-4. PMID:28878275 doi:http://dx.doi.org/10.1038/s41598-017-10944-4
- ↑ Belevich I, Borisov VB, Verkhovsky MI. Discovery of the true peroxy intermediate in the catalytic cycle of terminal oxidases by real-time measurement. J Biol Chem. 2007 Sep 28;282(39):28514-9. doi: 10.1074/jbc.M705562200. Epub 2007 , Aug 9. PMID:17690093 doi:http://dx.doi.org/10.1074/jbc.M705562200
- ↑ Giuffre A, Borisov VB, Arese M, Sarti P, Forte E. Cytochrome bd oxidase and bacterial tolerance to oxidative and nitrosative stress. Biochim Biophys Acta. 2014 Jul;1837(7):1178-87. doi:, 10.1016/j.bbabio.2014.01.016. Epub 2014 Jan 31. PMID:24486503 doi:http://dx.doi.org/10.1016/j.bbabio.2014.01.016
- ↑ 6.00 6.01 6.02 6.03 6.04 6.05 6.06 6.07 6.08 6.09 6.10 6.11 Thesseling A, Rasmussen T, Burschel S, Wohlwend D, Kagi J, Muller R, Bottcher B, Friedrich T. Homologous bd oxidases share the same architecture but differ in mechanism. Nat Commun. 2019 Nov 13;10(1):5138. doi: 10.1038/s41467-019-13122-4. PMID:31723136 doi:http://dx.doi.org/10.1038/s41467-019-13122-4
- ↑ 7.0 7.1 7.2 7.3 7.4 Safarian S, Rajendran C, Muller H, Preu J, Langer JD, Ovchinnikov S, Hirose T, Kusumoto T, Sakamoto J, Michel H. Structure of a bd oxidase indicates similar mechanisms for membrane-integrated oxygen reductases. Science. 2016 Apr 29;352(6285):583-6. doi: 10.1126/science.aaf2477. PMID:27126043 doi:http://dx.doi.org/10.1126/science.aaf2477
- ↑ Moosa A, Lamprecht DA, Arora K, Barry CE 3rd, Boshoff HIM, Ioerger TR, Steyn AJC, Mizrahi V, Warner DF. Susceptibility of Mycobacterium tuberculosis Cytochrome bd Oxidase Mutants to Compounds Targeting the Terminal Respiratory Oxidase, Cytochrome c. Antimicrob Agents Chemother. 2017 Sep 22;61(10). pii: AAC.01338-17. doi:, 10.1128/AAC.01338-17. Print 2017 Oct. PMID:28760899 doi:http://dx.doi.org/10.1128/AAC.01338-17
- ↑ 9.0 9.1 Hughes ER, Winter MG, Duerkop BA, Spiga L, Furtado de Carvalho T, Zhu W, Gillis CC, Buttner L, Smoot MP, Behrendt CL, Cherry S, Santos RL, Hooper LV, Winter SE. Microbial Respiration and Formate Oxidation as Metabolic Signatures of Inflammation-Associated Dysbiosis. Cell Host Microbe. 2017 Feb 8;21(2):208-219. doi: 10.1016/j.chom.2017.01.005. PMID:28182951 doi:http://dx.doi.org/10.1016/j.chom.2017.01.005
- ↑ 10.0 10.1 Shepherd M, Achard ME, Idris A, Totsika M, Phan MD, Peters KM, Sarkar S, Ribeiro CA, Holyoake LV, Ladakis D, Ulett GC, Sweet MJ, Poole RK, McEwan AG, Schembri MA. The cytochrome bd-I respiratory oxidase augments survival of multidrug-resistant Escherichia coli during infection. Sci Rep. 2016 Oct 21;6:35285. doi: 10.1038/srep35285. PMID:27767067 doi:http://dx.doi.org/10.1038/srep35285
- ↑ 11.0 11.1 Arora K, Ochoa-Montano B, Tsang PS, Blundell TL, Dawes SS, Mizrahi V, Bayliss T, Mackenzie CJ, Cleghorn LA, Ray PC, Wyatt PG, Uh E, Lee J, Barry CE 3rd, Boshoff HI. Respiratory flexibility in response to inhibition of cytochrome C oxidase in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2014 Nov;58(11):6962-5. doi: 10.1128/AAC.03486-14., Epub 2014 Aug 25. PMID:25155596 doi:http://dx.doi.org/10.1128/AAC.03486-14
- ↑ Galvan AE, Chalon MC, Rios Colombo NS, Schurig-Briccio LA, Sosa-Padilla B, Gennis RB, Bellomio A. Microcin J25 inhibits ubiquinol oxidase activity of purified cytochrome bd-I from Escherichia coli. Biochimie. 2019 May;160:141-147. doi: 10.1016/j.biochi.2019.02.007. Epub 2019 Feb, 19. PMID:30790617 doi:http://dx.doi.org/10.1016/j.biochi.2019.02.007
- ↑ Lu P, Heineke MH, Koul A, Andries K, Cook GM, Lill H, van Spanning R, Bald D. The cytochrome bd-type quinol oxidase is important for survival of Mycobacterium smegmatis under peroxide and antibiotic-induced stress. Sci Rep. 2015 May 27;5:10333. doi: 10.1038/srep10333. PMID:26015371 doi:http://dx.doi.org/10.1038/srep10333
Student Contributors
- Grace Bassler
- Emily Neal
- Marisa Villarreal
![Figure 1. Cartoon model of cytochrome bd-oxidase in E. coli. Dashed lines represent borders of cytoplasmic and periplasmic regions. A bound quinol between transmembrane helices 6 and 7 undergoes oxidation and releases protons into the periplasmic space, generating a proton gradient. Protons and oxygen atoms from the cytoplasmic side enter cytochrome bd oxidase through specific channels. Oxygen is reduced to water, which is released into the cytoplasmic space. Blue = CydA; green = CydB; yellow = CydX; pink = CydS. [PDB: 6RX4]](/wiki/images/thumb/a/a4/Pp_and_cp_of_oxdiase.png/550px-Pp_and_cp_of_oxdiase.png)
![Figure 3. H and o-channels of cytochrome bd-oxidase in E. coli. Channels are outlined in gray, water is shown as spheres, and various amino acids are labeled above. [PDB:6RX4]](/wiki/images/thumb/8/82/O_AND_H_CHANNEL.png/300px-O_AND_H_CHANNEL.png)

