We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
== Structural highlights == | == Structural highlights == | ||
===Substrate Structure=== | ===Substrate Structure=== | ||
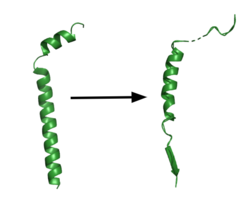
| - | [[Image:App.png|250 px|right|thumb| | + | [[Image:App.png|250 px|right|thumb|'''Figure 1. APP fragment conformational change in gamma secretase.''' APP bound to gamma secretase undergoes a conformational change. The free state consists of 2 helices. The N-terminal helix unfolds into a coil and the C-terminal helix unwinds into a β-strand. This β-strand interacts with PS1 and is the site of cleavage by gamma secretase.]] |
Though GS has multiple substrates, the substrate of main concern is called [https://en.wikipedia.org/wiki/Amyloid_precursor_protein Amyloid Precursor Protein (APP).] APP is composed of an N-terminal loop, a transmembrane (TM) helix, and a C-terminal β-strand. The uses lateral diffusion as a mechanism of entry into the enzyme, and once in place, the TM helix is anchored by hydrogen bonds. In order to differentiate substrates, the β-strand is often the main point of identification for the enzyme. After this, the helix undergoes unwinding and the process of catalysis can begin<ref name="Zhou">PMID:30630874</ref>. | Though GS has multiple substrates, the substrate of main concern is called [https://en.wikipedia.org/wiki/Amyloid_precursor_protein Amyloid Precursor Protein (APP).] APP is composed of an N-terminal loop, a transmembrane (TM) helix, and a C-terminal β-strand. The uses lateral diffusion as a mechanism of entry into the enzyme, and once in place, the TM helix is anchored by hydrogen bonds. In order to differentiate substrates, the β-strand is often the main point of identification for the enzyme. After this, the helix undergoes unwinding and the process of catalysis can begin<ref name="Zhou">PMID:30630874</ref>. | ||
Revision as of 01:42, 20 April 2020
Gamma Secretase
| |||||||||||
References
- ↑ Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 2.0 2.1 2.2 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
Student Contributors
Layla Wisser
Daniel Mulawa