We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1627
From Proteopedia
(Difference between revisions)
| Line 11: | Line 11: | ||
====Alpha Subunits==== | ====Alpha Subunits==== | ||
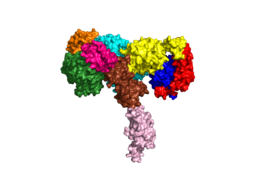
[[Image:Harrison Image2.png|thumb|right|260px|Figure 1: Insulin receptor apo receptor. Site L1' is colored a dark green, CR' is orange, L2' is bright blue, L2 is yellow, CR is red, L1 is dark blue, FnIII-1 is brown, and FnIII-2 is light pink. Insulin is shown bound and is colored dark pink. [http://www.rcsb.org/structure/6CE7 PDB 6CE7]]] | [[Image:Harrison Image2.png|thumb|right|260px|Figure 1: Insulin receptor apo receptor. Site L1' is colored a dark green, CR' is orange, L2' is bright blue, L2 is yellow, CR is red, L1 is dark blue, FnIII-1 is brown, and FnIII-2 is light pink. Insulin is shown bound and is colored dark pink. [http://www.rcsb.org/structure/6CE7 PDB 6CE7]]] | ||
| - | The alpha subunits make up the extracellular domain ([http://en.wikipedia.org/wiki/Ectodomain ectodomain]) of the insulin receptor and are the sites of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a <scene name='83/832953/Alpha_c_helix/6'>C-Terminal alpha helix</scene>(Figure 1). <ref name="Scapin"> PMID 29512653 </ref> The CT-alpha helix is unique in its position that allows it to reach across the receptor and interact with the insulin at the binding site on the opposing side of the receptor. The alpha subunits are held together by a [http://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='83/832953/Cysteine_bond/2'>cysteine residues</scene> at the CYS524 position on each alpha subunit. The disulfide bonds are important to the overall stabilization of the molecule | + | The alpha subunits make up the extracellular domain ([http://en.wikipedia.org/wiki/Ectodomain ectodomain]) of the insulin receptor and are the sites of insulin binding. The alpha subunit is comprised of two Leucine rich domains (L1 & L2), a Cysteine rich domain (CR), and a <scene name='83/832953/Alpha_c_helix/6'>C-Terminal alpha helix</scene> (Figure 1). <ref name="Scapin"> PMID 29512653 </ref> The CT-alpha helix is unique in its position that allows it to reach across the receptor and interact with the insulin at the binding site on the opposing side of the receptor. The alpha subunits are held together by a [http://en.wikipedia.org/wiki/Disulfide disulfide bond] between <scene name='83/832953/Cysteine_bond/2'>cysteine residues</scene> at the CYS524 position on each alpha subunit. The disulfide bonds are important to the overall stabilization of the molecule as it binds to insulin. Two types of insulin binding sites are present in the alpha subunits, <scene name='83/832953/Sites_1_and_1_prime_location/17'>sites 1 and 1'</scene> and <scene name='83/832953/Sites_2_and_2_prime_location/13'>sites 2 and 2'</scene> (Figure 2). The sites are in pairs because of the heterodimeric nature of the receptor. Due to structural differences, as well as greater surface area and accessibility, binding sites 1 and 1' have much higher affinity than that of sites 2 and 2'. Insulin can also bind at sites 2 and 2', but the location on the back of the beta sheet of the FnIII-1 domain and lack of surface area decreases the likelihood of their binding site becoming occupied as quickly. <ref name="Uchikawa"> DOI 10.7554/eLife.48630 </ref> [http://en.wikipedia.org/wiki/Transmission_electron_cryomicroscopy Cryo-EM] has imaged insulin bound structures that displayed a T-shape conformation in the alpha subunits, which make up the receptors extracellular domain region.<ref name="Uchikawa" /> |
[[Image:4 sites highlighted - Harrison.png|thumb|right|260px|Figure 2: The four binding sites of insulin. Sites 1 and 1' are colored green, sites 2 and 2' are colored red. [http://www.rcsb.org/structure/6SOF PDB 6SOF]]] | [[Image:4 sites highlighted - Harrison.png|thumb|right|260px|Figure 2: The four binding sites of insulin. Sites 1 and 1' are colored green, sites 2 and 2' are colored red. [http://www.rcsb.org/structure/6SOF PDB 6SOF]]] | ||
Revision as of 15:37, 20 April 2020
Homo sapiens Insulin Receptor
| |||||||||||
References
- ↑ 1.0 1.1 De Meyts P. The Insulin Receptor and Its Signal Transduction Network PMID:27512793
- ↑ 2.0 2.1 2.2 2.3 Tatulian SA. Structural Dynamics of Insulin Receptor and Transmembrane Signaling. Biochemistry. 2015 Sep 15;54(36):5523-32. doi: 10.1021/acs.biochem.5b00805. Epub , 2015 Sep 3. PMID:26322622 doi:http://dx.doi.org/10.1021/acs.biochem.5b00805
- ↑ Hubbard SR. Crystal structure of the activated insulin receptor tyrosine kinase in complex with peptide substrate and ATP analog. EMBO J. 1997 Sep 15;16(18):5572-81. PMID:9312016 doi:10.1093/emboj/16.18.5572
- ↑ 4.0 4.1 Scapin G, Dandey VP, Zhang Z, Prosise W, Hruza A, Kelly T, Mayhood T, Strickland C, Potter CS, Carragher B. Structure of the Insulin Receptor-Insulin Complex by Single Particle CryoEM analysis. Nature. 2018 Feb 28. pii: nature26153. doi: 10.1038/nature26153. PMID:29512653 doi:http://dx.doi.org/10.1038/nature26153
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 Uchikawa E, Choi E, Shang G, Yu H, Bai XC. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor-ligand complex. Elife. 2019 Aug 22;8. pii: 48630. doi: 10.7554/eLife.48630. PMID:31436533 doi:http://dx.doi.org/10.7554/eLife.48630
- ↑ Cabail MZ, Li S, Lemmon E, Bowen ME, Hubbard SR, Miller WT. The insulin and IGF1 receptor kinase domains are functional dimers in the activated state. Nat Commun. 2015 Mar 11;6:6406. doi: 10.1038/ncomms7406. PMID:25758790 doi:http://dx.doi.org/10.1038/ncomms7406
- ↑ 7.0 7.1 White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1-4. PMID:8276779
- ↑ McKern NM, Lawrence MC, Streltsov VA, Lou MZ, Adams TE, Lovrecz GO, Elleman TC, Richards KM, Bentley JD, Pilling PA, Hoyne PA, Cartledge KA, Pham TM, Lewis JL, Sankovich SE, Stoichevska V, Da Silva E, Robinson CP, Frenkel MJ, Sparrow LG, Fernley RT, Epa VC, Ward CW. Structure of the insulin receptor ectodomain reveals a folded-over conformation. Nature. 2006 Sep 14;443(7108):218-21. Epub 2006 Sep 6. PMID:16957736 doi:10.1038/nature05106
- ↑ Weis F, Menting JG, Margetts MB, Chan SJ, Xu Y, Tennagels N, Wohlfart P, Langer T, Muller CW, Dreyer MK, Lawrence MC. The signalling conformation of the insulin receptor ectodomain. Nat Commun. 2018 Oct 24;9(1):4420. doi: 10.1038/s41467-018-06826-6. PMID:30356040 doi:http://dx.doi.org/10.1038/s41467-018-06826-6
- ↑ Boucher J, Kleinridders A, Kahn CR. Insulin receptor signaling in normal and insulin-resistant states. Cold Spring Harb Perspect Biol. 2014 Jan 1;6(1). pii: 6/1/a009191. doi:, 10.1101/cshperspect.a009191. PMID:24384568 doi:http://dx.doi.org/10.1101/cshperspect.a009191
- ↑ Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. 2005 May;26(2):19-39. PMID:16278749
- ↑ Riddle MC. Treatment of diabetes with insulin. From art to science. West J Med. 1983 Jun;138(6):838-46. PMID:6351440
Student Contributors
- Harrison Smith
- Alyssa Ritter