We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1626
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
===Mitochondrial Calcium Uniporter Structure=== | ===Mitochondrial Calcium Uniporter Structure=== | ||
| - | The MCU was originally thought to be composed of a [https://en.wikipedia.org/wiki/Pentamer pentamer] of five identical subunits, but it is now known to exist as a [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimer] of <scene name='83/832952/Dimer_of_dimers/5'>dimers</scene>.<ref name="Woods">PMID:31869674</ref> More specifically, it is composed of two [https://en.wikipedia.org/wiki/Coiled_coil coiled-coil] domains and two transmembrane domains.<ref name="Woods"/> The hydrophobic transmembrane domain is located in the inner mitochondrial membrane ([https://en.wikipedia.org/wiki/Inner_mitochondrial_membrane IMM]) and the hydrophilic coiled-coil domain exists in the mitochondrial matrix.<ref name="Baradaran"/> The transmembrane domain (TMD) consists of eight separate helices (TM1 and TM2 from each subunit) that are connected by mostly hydrophobic amino acids in the IMS and has four-fold symmetry.<ref name="Baradaran"/> <scene name='83/832952/Tm1/2'>TM1</scene> packs tightly against <scene name='83/832952/Tm2/2'>TM2</scene> from the neighboring subunit which conveys a sense of domain-swapping.<ref name="Fan">PMID:29995856</ref> This section of the MCU can be roughly divided into a narrow outer leaflet portion with the selectivity filter and lined by the <scene name='83/832952/Tm2/2'>TM2</scene> helices and a wide inner leaflet.<ref name="Baradaran"/> Past the transmembrane domain, the N-terminal domains of the <scene name='83/832952/Tm1/2'>TM1</scene> helices extend into the matrix and form coiled-coils with a C-terminal helix.<ref name="Baradaran"/> These "legs" are separated from each other which allows enough space for calcium ions to diffuse out into the matrix.<ref name="Baradaran"/> Additionally, this domain is responsible for assembly of the MCU and [https://en.wikipedia.org/wiki/Post-translational_modification post-translational modification].<ref name="Fan"/> Finally, each leg ends in a non-translated domain (NTD).<ref name="Baradaran"/> While the MCU can intake calcium without the NTD, it may have regulatory functions and the ability to bend transmembrane helices to constrict the pore.<ref name="Baradaran"/> <ref name="Fan"/> | + | The MCU was originally thought to be composed of a [https://en.wikipedia.org/wiki/Pentamer pentamer] of five identical subunits, but it is now known to exist as a [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimer] of <scene name='83/832952/Dimer_of_dimers/5'>dimers</scene>.<ref name="Woods">PMID:31869674</ref> More specifically, it is composed of two [https://en.wikipedia.org/wiki/Coiled_coil coiled-coil] domains and two transmembrane domains.<ref name="Woods"/> The hydrophobic '''transmembrane domain''' is located in the inner mitochondrial membrane ([https://en.wikipedia.org/wiki/Inner_mitochondrial_membrane IMM]) and the hydrophilic '''coiled-coil domain''' exists in the mitochondrial matrix.<ref name="Baradaran"/> The '''transmembrane domain''' (TMD) consists of eight separate helices ('''TM1''' and '''TM2''' from each subunit) that are connected by mostly hydrophobic amino acids in the IMS and has four-fold symmetry.<ref name="Baradaran"/> <scene name='83/832952/Tm1/2'>TM1</scene> packs tightly against <scene name='83/832952/Tm2/2'>TM2</scene> from the neighboring subunit which conveys a sense of domain-swapping.<ref name="Fan">PMID:29995856</ref> This section of the MCU can be roughly divided into a narrow outer leaflet portion with the '''selectivity filter''' and lined by the <scene name='83/832952/Tm2/2'>TM2</scene> helices and a wide inner leaflet.<ref name="Baradaran"/> Past the transmembrane domain, the N-terminal domains of the <scene name='83/832952/Tm1/2'>TM1</scene> helices extend into the matrix and form coiled-coils with a C-terminal helix.<ref name="Baradaran"/> These "legs" are separated from each other which allows enough space for calcium ions to diffuse out into the matrix.<ref name="Baradaran"/> Additionally, this domain is responsible for assembly of the MCU and [https://en.wikipedia.org/wiki/Post-translational_modification post-translational modification].<ref name="Fan"/> Finally, each leg ends in a '''non-translated domain''' (NTD).<ref name="Baradaran"/> While the MCU can intake calcium without the NTD, it may have regulatory functions and the ability to bend transmembrane helices to constrict the pore.<ref name="Baradaran"/> <ref name="Fan"/> |
===Selectivity Filter=== | ===Selectivity Filter=== | ||
| Line 33: | Line 33: | ||
===Mutations=== | ===Mutations=== | ||
| - | There are a number of mutations that completely eliminate calcium uptake by the MCU. For example, mutation of [https://en.wikipedia.org/wiki/Tryptophan W], [https://en.wikipedia.org/wiki/Aspartic_acid D], [https://en.wikipedia.org/wiki/Glutamic_acid E], or [https://en.wikipedia.org/wiki/Proline P] of the WDXXEP motif altered the highly conserved selectivity filter and completely eliminated calcium uptake.<ref name="Baradaran"/><ref name="Fan"/> For example, even mutating Glu228 to an aspartate significantly changed the dimensions of the pore and inhibited uptake of calcium.<ref name="Baradaran"/> However, mutation of either X residue was not detrimental to calcium uptake.<ref name="Baradaran"/> Furthermore, mutation of a tyrosine residue directly below the selectivity filter substantially impaired calcium intake and proper protein folding.<ref name="Fan"/> The residue on TM1 that affected calcium uptake the most in human MCU was Trp317 which has a side chain constituting a primary contact point between TM1 and TM2.<ref name="Fan"/> Mutation of Phe326 or Gly331 of the TM1-TM2 linker in human MCU affected the linker conformation and configuration of the pore entrance and impaired calcium intake.<ref name="Fan"/> | + | There are a number of mutations that completely eliminate calcium uptake by the MCU. For example, mutation of [https://en.wikipedia.org/wiki/Tryptophan W], [https://en.wikipedia.org/wiki/Aspartic_acid D], [https://en.wikipedia.org/wiki/Glutamic_acid E], or [https://en.wikipedia.org/wiki/Proline P] of the WDXXEP motif altered the highly conserved selectivity filter and completely eliminated calcium uptake.<ref name="Baradaran"/><ref name="Fan"/> For example, even mutating Glu228 to an aspartate significantly changed the dimensions of the pore and inhibited uptake of calcium.<ref name="Baradaran"/> However, mutation of either X residue was not detrimental to calcium uptake.<ref name="Baradaran"/> Furthermore, mutation of a tyrosine residue directly below the selectivity filter substantially impaired calcium intake and proper protein folding.<ref name="Fan"/> The residue on TM1 that affected calcium uptake the most in human MCU was '''Trp317 (Trp210)''' which has a side chain constituting a primary contact point between TM1 and TM2.<ref name="Fan"/> Mutation of '''Phe326 (Phe218)''' or '''Gly331 (Gly223)''' of the TM1-TM2 linker in human MCU affected the linker conformation and configuration of the pore entrance and impaired calcium intake.<ref name="Fan"/> |
==Regulation and Inhibition== | ==Regulation and Inhibition== | ||
Revision as of 23:49, 20 April 2020
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
Mitochondrial Calcium Uniporter (MCU) Complex
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0331-8. doi:, 10.1038/s41586-018-0331-8. PMID:29995857 doi:http://dx.doi.org/10.1038/s41586-018-0331-8
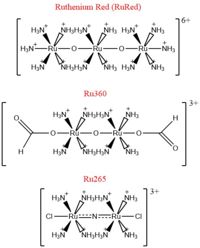
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Woods JJ, Wilson JJ. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr Opin Chem Biol. 2019 Dec 20;55:9-18. doi: 10.1016/j.cbpa.2019.11.006. PMID:31869674 doi:http://dx.doi.org/10.1016/j.cbpa.2019.11.006
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017 Sep 7;24(1):70. doi: 10.1186/s12929-017-0375-3. PMID:28882140 doi:http://dx.doi.org/10.1186/s12929-017-0375-3
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9
Student Contributors
Ryan Heumann
Rieser Wells