We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1619
From Proteopedia
(Difference between revisions)
| Line 28: | Line 28: | ||
===Active Site=== | ===Active Site=== | ||
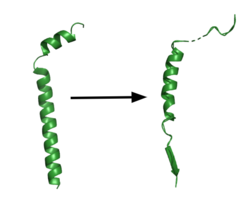
| - | The <scene name='83/832945/Asp_257_and_asp_385/12'>active site</scene> is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Both TM6 and TM7 contribute an aspartate residue to the active site. These two aspartates, <scene name='83/832945/Asp_257_and_asp_385/10'>Asp257 and Asp385</scene> are located approximately 10.6 A˚ apart when inactive.<ref name="Bai">PMID:26280335</ref> Substrate recognition is controlled by the closely spaced PAL sequence of <scene name='83/832945/Asp_257_and_asp_385/11'>Pro433, Ala434, and Leu435</scene>. GS becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate. Two β-strands are induced in PS1, creating an <scene name='83/832945/Beta_sheet_complex/1'>antiparallel β-sheet</scene> with the β-strand of the substrate.<ref name="Zhou" /> The β-strand of the substrate interacts via main chain H-bonds with the PAL sequence, stabilizing the active site. Asp257 and Asp385 hydrogen bond to each other and are located 6–7 Å away from the scissile peptide bond of the substrate, allowing catalysis to occur.<ref name="Yang" /> | + | The <scene name='83/832945/Asp_257_and_asp_385/12'>active site</scene> is located between TM6 and TM7 of the PS1 subunit, which is mainly hydrophilic and disordered. Both TM6 and TM7 contribute an aspartate residue to the active site. These two aspartates, <scene name='83/832945/Asp_257_and_asp_385/10'>Asp257 and Asp385</scene> are located approximately 10.6 A˚ apart when inactive.<ref name="Bai">PMID:26280335</ref> Substrate recognition is controlled by the closely spaced PAL sequence of <scene name='83/832945/Asp_257_and_asp_385/11'>Pro433, Ala434, and Leu435</scene>. GS becomes active upon substrate binding, when TM2 and TM6 each rotate about 15 degrees to more closely associate. Two β-strands are induced in PS1, creating an <scene name='83/832945/Beta_sheet_complex/1'>antiparallel β-sheet</scene> with the β-strand of the substrate.<ref name="Zhou" /> The β-strand of the substrate interacts via main chain H-bonds with the PAL sequence, stabilizing the active site. Asp257 and Asp385 hydrogen bond to each other and are located 6–7 Å away from the scissile peptide bond of the substrate, allowing catalysis to occur.<ref name="Yang" /> GS cleaves in 3 residue segments which is driven by the presence of three amino acid binding pockets in the active site. <ref name="Bolduc" /> |
| + | In APP, the cleavage site is between the helix and the N-terminal β-strand. GS can cleave via different pathways, depending on its starting point, but the 2 most commonly used pathways produce Aβ48 and Aβ49.<ref name="Bolduc">PMID:27580372</ref>. Tripeptide cleavage starting between <scene name='83/832945/3_residues_for_cleavage/2'>Thr719 and Leu720</scene> results in Aβ48. Cleavage between <scene name='83/832945/3_residues_for_cleavage/3'>Leu720 and Val721</scene> yields Aβ49. The accumulation of these Aβ peptides has strong implications in Alzheimer's disease.<ref name="Zhou">PMID:30630874</ref> | ||
Revision as of 01:28, 21 April 2020
Gamma Secretase
| |||||||||||
References
- ↑ 1.0 1.1 Yang G, Zhou R, Shi Y. Cryo-EM structures of human gamma-secretase. Curr Opin Struct Biol. 2017 Oct;46:55-64. doi: 10.1016/j.sbi.2017.05.013. Epub, 2017 Jul 17. PMID:28628788 doi:http://dx.doi.org/10.1016/j.sbi.2017.05.013
- ↑ 2.0 2.1 2.2 2.3 Zhou R, Yang G, Guo X, Zhou Q, Lei J, Shi Y. Recognition of the amyloid precursor protein by human gamma-secretase. Science. 2019 Feb 15;363(6428). pii: science.aaw0930. doi:, 10.1126/science.aaw0930. Epub 2019 Jan 10. PMID:30630874 doi:http://dx.doi.org/10.1126/science.aaw0930
- ↑ 3.0 3.1 Bai XC, Yan C, Yang G, Lu P, Ma D, Sun L, Zhou R, Scheres SH, Shi Y. An atomic structure of human gamma-secretase. Nature. 2015 Aug 17. doi: 10.1038/nature14892. PMID:26280335 doi:http://dx.doi.org/10.1038/nature14892
- ↑ 4.0 4.1 Bolduc DM, Montagna DR, Seghers MC, Wolfe MS, Selkoe DJ. The amyloid-beta forming tripeptide cleavage mechanism of gamma-secretase. Elife. 2016 Aug 31;5. doi: 10.7554/eLife.17578. PMID:27580372 doi:http://dx.doi.org/10.7554/eLife.17578
- ↑ Kumar D, Ganeshpurkar A, Kumar D, Modi G, Gupta SK, Singh SK. Secretase inhibitors for the treatment of Alzheimer's disease: Long road ahead. Eur J Med Chem. 2018 Mar 25;148:436-452. doi: 10.1016/j.ejmech.2018.02.035. Epub , 2018 Feb 15. PMID:29477076 doi:http://dx.doi.org/10.1016/j.ejmech.2018.02.035
Student Contributors
Layla Wisser
Daniel Mulawa