We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1607
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
===Selectivity Filter=== | ===Selectivity Filter=== | ||
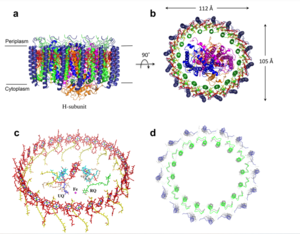
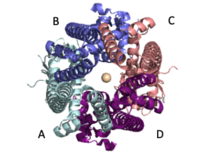
| - | The <scene name='83/832933/Selectivity_filter/3'>selectivity filter</scene> contains <scene name='83/832933/Glu_residues_all/1'>Glu358</scene>, Trp354, and Pro359 to allow calcium to pass through the uniporter. The carboxylate oxygen of the <scene name='83/832933/Glu_358/3'>Glu358</scene> side chains draw in the positive calcium ion. The <scene name='83/832933/Diameter/2'>diameter</scene> of the carboxyl ring is about 4Å, allowing only a dehydrated Ca2+ ion to bind. Trp38, which is directly next to the Glu residues, stabilizes the carbonyl side chains through <scene name='83/832933/H_bond_trp354_glu358/3'>hydrogen bonding</scene> and anion pi interactions. These Trp residues also form stacking interactions with Pro359, which orientate the Glu carboxyl side chains towards the middle of the pore to interact with Ca2+ ions. <ref name=”Yoo”>PMID:29954988</ref> | + | The <scene name='83/832933/Selectivity_filter/3'>selectivity filter</scene> contains <scene name='83/832933/Glu_residues_all/1'>Glu358</scene>, <scene name='83/832933/Trp354/1'>Trp354</scene>, and <scene name='83/832933/Pro359/1'>Pro359</scene> to allow calcium to pass through the uniporter. The carboxylate oxygen of the <scene name='83/832933/Glu_358/3'>Glu358</scene> side chains draw in the positive calcium ion. The <scene name='83/832933/Diameter/2'>diameter</scene> of the carboxyl ring is about 4Å, allowing only a dehydrated Ca2+ ion to bind. Trp38, which is directly next to the Glu residues, stabilizes the carbonyl side chains through <scene name='83/832933/H_bond_trp354_glu358/3'>hydrogen bonding</scene> and anion pi interactions. These Trp residues also form stacking interactions with Pro359, which orientate the Glu carboxyl side chains towards the middle of the pore to interact with Ca2+ ions. <ref name=”Yoo”>PMID:29954988</ref> |
[http://www.rcsb.org/structure/6DT0 Calcium Uniporter Structure] | [http://www.rcsb.org/structure/6DT0 Calcium Uniporter Structure] | ||
Revision as of 02:37, 21 April 2020
Mitochondrial Calcium Uniporter
| |||||||||||
References
- ↑ 1.0 1.1 1.2 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 2.0 2.1 2.2 2.3 2.4 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9
- ↑ Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018 Jun 28. pii: science.aar4056. doi: 10.1126/science.aar4056. PMID:29954988 doi:http://dx.doi.org/10.1126/science.aar4056