Davis L. Martinec/Sandbox 4eqv
From Proteopedia
(Difference between revisions)
| Line 5: | Line 5: | ||
== General Description == | == General Description == | ||
| - | 4EQV is 469 kDa invertase(SInv) isolated from Saccharomyces cerevisiae. SInv catalyzes the hydrolysis of sucrose into fructose and glucose, thus making it an essential enzyme to plants and other organisms, such as honey bees<ref name="3d">DOI: 10.1074/jbc.M112.446435 </ref>. Invertase also lends itself to industry and is extensively used fermentation, where yeast are employed to process sugar into ethanol. | + | 4EQV is 469 kDa invertase(SInv) isolated from Saccharomyces cerevisiae. SInv catalyzes the hydrolysis of sucrose into fructose and glucose, thus making it an essential enzyme to plants and other organisms, such as honey bees<ref name="3d">DOI: 10.1074/jbc.M112.446435 </ref>. Invertase also lends itself to industry and is extensively used in fermentation, where yeast are employed to process sugar into ethanol. |
| - | Yeast invertase was first isolated in 1860 by Berthelot, but had been alluded to by Dubrunfaut in 1847.<ref>DOI: 10.1074/jbc.M112.446435 </ref> Invertase was posited to be an intracellular | + | Yeast invertase was first isolated in 1860 by Berthelot, but had been alluded to by Dubrunfaut in 1847.<ref>DOI: 10.1074/jbc.M112.446435 </ref> Invertase was posited to be an intracellular enzyme, yet de la Fuente and Sols were able to show that invertase can be excreted by the cell, thus classifying it as extracellular<ref>https://doi.org/10.1016/0006-3002(62)90526-7</ref>. The intracellular form of the enzyme is not glycosylated, while the extracellular form is extensively glycosylated<ref name="3d" />. The same gene codes for glycosylated and non-glycosylated forms of the enzyme they differ when being transcribed into mRNA, and the extracellular bound SInv is tagged with a signal peptide.<ref>https://doi.org/10.1007/BF00425540</ref> |
| Line 16: | Line 16: | ||
=== Catalytic β-propeller Domain === | === Catalytic β-propeller Domain === | ||
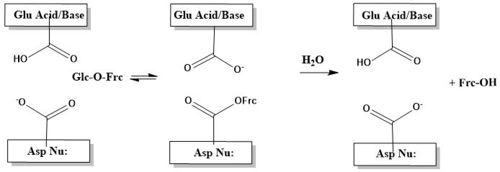
| - | The <scene name='84/842891/Beta-propeller_domain/4'>β-propeller domain</scene> of 4EQV, shown in crimson, is the catalytic domain of the monomer. The domain is composed mostly of antiparallel β-strands which form five blades, each containing four antiparallel β-strands. The <scene name='84/842891/Closed_active_site/2'>active site</scene> of the β-propeller domain is formed at the axis of the five blades. This catalytic pocket | + | The <scene name='84/842891/Beta-propeller_domain/4'>β-propeller domain</scene> of 4EQV, shown in crimson, is the catalytic domain of the monomer. The domain is composed mostly of antiparallel β-strands which form five blades, each containing four antiparallel β-strands. The <scene name='84/842891/Closed_active_site/2'>active site</scene> of the β-propeller domain is formed at the axis of the five blades. This catalytic pocket contains nucleophilic residue Asp22 at its base and is lined with multiple hydrophobic residues, namely Trp48, Phe82, Trp291, Phe296, and Phe388. The <scene name='84/842891/Closed_pocket_with_nucleophile/2'>catalytic pockets</scene> of the A/B and C/D chains are rather specific for sucrose. This specificity is due to Gln201, which binds to sucrose and Asp228 conveys an affinity for glucose moiety<ref name="fructo">DOI: 10.1128/AEM.05032-11</ref>. |
| - | The catalytic process | + | The catalytic process is two-step. First, there is the nucleophilic attack of the anomeric carbon of the fructose moiety by Asp22, to form a covalent enzyme-substrate complex<ref name="fructo" />. Glucose, the leaving group, is simultaneously protonated by Glu203, which then deprontonates the "acceptor" molecule to activate it as a nucleophile, which then releases fructose<ref name="fructo" />. The figure below illustrates this process using a cartoon. |
[[Image:Catalysis_4eqv_mech.JPG| 500 px]] | [[Image:Catalysis_4eqv_mech.JPG| 500 px]] | ||
| Line 30: | Line 30: | ||
=== Open & Closed Assembly === | === Open & Closed Assembly === | ||
| - | The β-sandwich domain section touches on the topic of dimerization and tetramerization, and that will be explored here further. SInv is an octamer, though is better described as a tetramer of dimers. The dimers can be further categorized into open(chains E, F, G, H) | + | The β-sandwich domain section touches on the topic of dimerization and tetramerization, and that will be explored here further. SInv is an octamer, though is better described as a tetramer of dimers. The dimers can be further categorized into open(chains E, F, G, H) and closed assemblies(chains A, B, C, D). The difference in assembly type - open versus closed - has implications involving substrate scope. The closed assembly is borne out of many polar interactions and large surface area of interactions between the β-sandwich domain of one chain and catalytic pocket(β-propeller domain) of the corresponding chain<ref>DOI: 10.1016/j.jmb.2007.05.022</ref>. Compared with the open assembly, the closed assembly has an interaction surface twice as large as the open assembly<ref name="fructo" />. This strong interaction between monomers makes the catalytic pocket highly specific for sucrose<ref name="fructo" />. The images below illustrate difference between the monomers that make up 4EQV. The upper left image shows the alignment of chains B and F, upper right shows the alignment of chains A and E. The differences highlighted here are responsible for the open vs. closed assemblies. The lower left image shows the alignment of chains E and F, lower right shows the alignment of chains A and B. |
<div style="display: inline; width: 700px; float: right;"> | <div style="display: inline; width: 700px; float: right;"> | ||
| Line 37: | Line 37: | ||
[[Image:Chain_E-F_alignment_ray_trace.png|350 px]][[Image:Chain_A-B_alignment_ray_trace.png|350 px]] </div> | [[Image:Chain_E-F_alignment_ray_trace.png|350 px]][[Image:Chain_A-B_alignment_ray_trace.png|350 px]] </div> | ||
| - | Though it | + | Though it may seem like a deficiency that only four of the eight catalytic sites are highly specific for sucrose or oligosaccharide with more than 4 units, this is actually allows 4EQV to much more versatile. The open assemblies(chains E, F, G, H), are capable of accommodating larger substrates such as ketoses or extended fructans. This can explain yeast's ability to to utilize the varied sugars found in its environment, and contributing to the the metabolic success of yeast<ref name="3d" />. |
== Evolutionary Conservation & Related Proteins == | == Evolutionary Conservation & Related Proteins == | ||
| Line 43: | Line 43: | ||
4EQV is an essential protein for microorganisms and plants. Its primary role is to catalyze the hydrolysis of sucrose and other small oligosaccharides into fructose and glucose. Most of the conserved residues are located in the β-propeller domain. The interior of the five blades of the β-propeller are highly conserved, with blade one the most conserved and blade four the least conserved<ref name="3d" />. The catalytic pocket of the enzyme is located within the five blades, so it reasonable that this section would be highly conserved. The β-sandwich domain shows relatively little conservation and this could have implications for the evolution of open and closed assemblies. | 4EQV is an essential protein for microorganisms and plants. Its primary role is to catalyze the hydrolysis of sucrose and other small oligosaccharides into fructose and glucose. Most of the conserved residues are located in the β-propeller domain. The interior of the five blades of the β-propeller are highly conserved, with blade one the most conserved and blade four the least conserved<ref name="3d" />. The catalytic pocket of the enzyme is located within the five blades, so it reasonable that this section would be highly conserved. The β-sandwich domain shows relatively little conservation and this could have implications for the evolution of open and closed assemblies. | ||
| - | <scene name='84/842891/Beta_prop_conservation/1'>This representation</scene> has been colored coded according to the scale below to show conserved and non conserved residues for a monomer that forms a closed assembly. | + | <scene name='84/842891/Beta_prop_conservation/1'>This representation</scene> has been colored coded according to the scale below to show conserved and non-conserved residues for a monomer that forms a closed assembly. |
{{Template:ColorKey_ConSurf}} | {{Template:ColorKey_ConSurf}} | ||
Revision as of 14:00, 28 April 2020
4EQV - Saccharomyces Invertase
| |||||||||||