We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Sumit Kamat/Sandbox Reserved 901
From Proteopedia
(Difference between revisions)
| Line 20: | Line 20: | ||
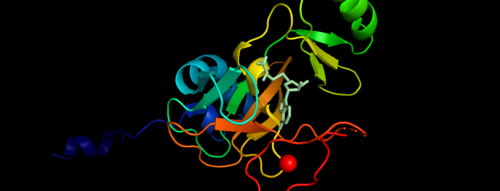
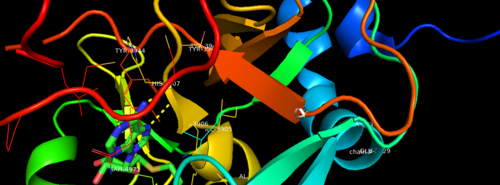
MLL-1 is a 431-kDa protein to be a structural and functional homolog of the Drosophila trithorax (TRX) protein. Two domains are highly conserved between MLL and TRX consist of a carboxy-terminal SET (Su(var)3-9, enhancer-of-zeste, and trithorax) domain and internal plant homeodomain (PHD) fingers. Both domains are found in many chromatin-associated transcriptional regulators and are thought to function either directly in chromatin modification or as protein-protein interaction surfaces for the recruitment of chromatin-modifying machinery <ref> https://doi.org/10.1182/blood-2002-04-1015 </ref>. | MLL-1 is a 431-kDa protein to be a structural and functional homolog of the Drosophila trithorax (TRX) protein. Two domains are highly conserved between MLL and TRX consist of a carboxy-terminal SET (Su(var)3-9, enhancer-of-zeste, and trithorax) domain and internal plant homeodomain (PHD) fingers. Both domains are found in many chromatin-associated transcriptional regulators and are thought to function either directly in chromatin modification or as protein-protein interaction surfaces for the recruitment of chromatin-modifying machinery <ref> https://doi.org/10.1182/blood-2002-04-1015 </ref>. | ||
| - | |||
| - | == Sequence Highlights == | ||
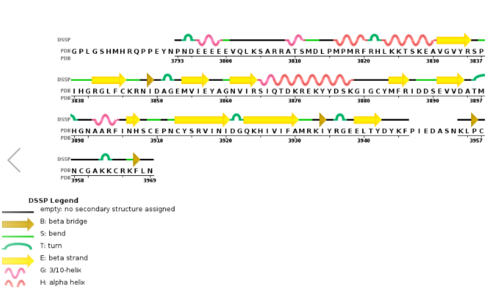
[[Image:2w5y sequence chain final.PNG|thumb|500px|center|Figure 3. Secondary structure of KMT2A SET Domain with the cofactor product S-Adenosylhomocysteine. <ref> PMID: 6667333 </ref> ]] | [[Image:2w5y sequence chain final.PNG|thumb|500px|center|Figure 3. Secondary structure of KMT2A SET Domain with the cofactor product S-Adenosylhomocysteine. <ref> PMID: 6667333 </ref> ]] | ||
| + | |||
| + | |||
| + | [[Image:Alignment of Kmt2a Domains.jpg|thumb|500px|center|Figure 4. Phylogenetic analysis based on the sequence of only the SET domains of KMT2 proteins in yeast and Humans <ref> Zhang, Y., Mittal, A., Reid, J., Reich, S., Gamblin, S. J., & Wilson, J. R. (2015). Evolving Catalytic Properties of the MLL Family SET Domain. Structure (London, England : 1993), 23(10), 1921–1933. https://doi.org/10.1016/j.str.2015.07.018</ref> ]] | ||
== Relevance == | == Relevance == | ||
Revision as of 19:15, 28 April 2020
Histone-lysine N-methyltransferase 2A KMT2A
| |||||||||||