User:Sumit Kamat/Sandbox Reserved 901
From Proteopedia
(Difference between revisions)
| Line 18: | Line 18: | ||
== Structural Analysis == | == Structural Analysis == | ||
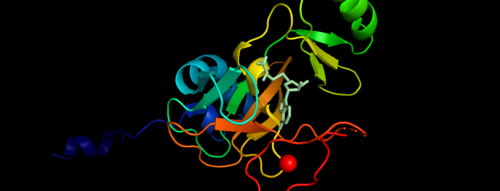
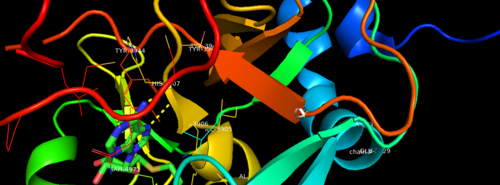
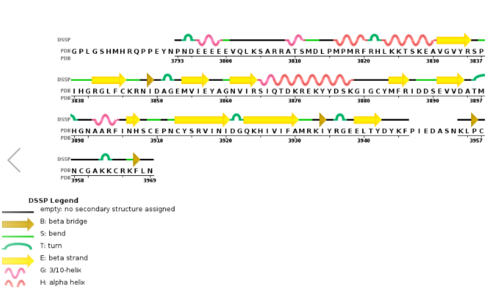
| - | MLL-1 is a 431-kDa protein to be a structural and functional homolog of the Drosophila trithorax (TRX) protein. Two domains are highly conserved between MLL and TRX consist of a carboxy-terminal SET (Su(var)3-9, enhancer-of-zeste, and trithorax) domain and internal plant homeodomain (PHD) fingers. Both domains are found in many chromatin-associated transcriptional regulators and are thought to function either directly in chromatin modification or as protein-protein interaction surfaces for the recruitment of chromatin-modifying machinery <ref> https://doi.org/10.1182/blood-2002-04-1015 </ref>. | + | MLL-1 is a 431-kDa protein to be a structural and functional homolog of the Drosophila trithorax (TRX) protein. Two domains are highly conserved between MLL and TRX consist of a carboxy-terminal SET (Su(var)3-9, enhancer-of-zeste, and trithorax) domain and internal plant homeodomain (PHD) fingers. Both domains are found in many chromatin-associated transcriptional regulators and are thought to function either directly in chromatin modification or as protein-protein interaction surfaces for the recruitment of chromatin-modifying machinery <ref> https://doi.org/10.1182/blood-2002-04-1015 </ref>.There are no significant changes in the structure of the SET domain of the binary complex with the cofactor binding in the surface pocket. |
| Line 26: | Line 26: | ||
[[Image:Alignment of Kmt2a Domains.jpg|thumb|500px|center|Figure 4. Phylogenetic analysis based on the sequence of only the SET domains of KMT2 proteins in yeast and Humans <ref> Zhang, Y., Mittal, A., Reid, J., Reich, S., Gamblin, S. J., & Wilson, J. R. (2015). Evolving Catalytic Properties of the MLL Family SET Domain. Structure (London, England : 1993), 23(10), 1921–1933. https://doi.org/10.1016/j.str.2015.07.018</ref> ]] | [[Image:Alignment of Kmt2a Domains.jpg|thumb|500px|center|Figure 4. Phylogenetic analysis based on the sequence of only the SET domains of KMT2 proteins in yeast and Humans <ref> Zhang, Y., Mittal, A., Reid, J., Reich, S., Gamblin, S. J., & Wilson, J. R. (2015). Evolving Catalytic Properties of the MLL Family SET Domain. Structure (London, England : 1993), 23(10), 1921–1933. https://doi.org/10.1016/j.str.2015.07.018</ref> ]] | ||
| - | == | + | == Clinical Significance == |
| + | KMT2A is an important gene because of its association with cognition and development. Epigenetic dysregulation of DNA methylation which leads to abnormal H3K4 trimethylation has been implicated in several neurological disorders such as autism <ref> Shulha HP, Cheung I, Whittle C, et al. Epigenetic Signatures of Autism: Trimethylated H3K4 Landscapes in Prefrontal Neurons. Arch Gen Psychiatry. 2012;69(3):314–324. doi:10.1001/archgenpsychiatry.2011.151 </ref>. In schizophrenia MLL-1 participates in the process of GAD67 downregulation resulting in decreased H3K4 methylation at GABAergic gene promoters <ref> Huang, H. S., Matevossian, A., Whittle, C., Kim, S. Y., Schumacher, A., Baker, S. P., & Akbarian, S. (2007). Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. The Journal of neuroscience : the official journal of the Society for Neuroscience, 27(42), 11254–11262. https://doi.org/10.1523/JNEUROSCI.3272-07.2007 </ref>. Rearrangements of the MLL1 gene are associated with aggressive acute leukemias, both lymphoblastic and myeloid.native MLL1 regulates Hox genes in hematopoietic cells to establish cellular identity (15, 16, 22). Disruption of MLL1 function by chromosomal translocation results in Hox misregulation coupled with the onset of leukemic phenotypes. Despite being an aggressive leukemia, the MLL1 rearranged sub-type had the lowest mutation rates reported for any cancer <ref> Guenther MG, Jenner RG, Chevalier B, et al. Global and Hox-specific roles for the MLL1 methyltransferase. Proc Natl Acad Sci U S A. 2005;102(24):8603–8608. doi:10.1073/pnas.0503072102 </ref>, <ref> Andersson AK, Ma J, Wang J, et al. The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nat Genet. 2015;47(4):330–337. doi:10.1038/ng.3230 </ref>. | ||
| + | Mutations in MLL1 cause Wiedemann-Steiner syndrome and Acute lymphoblastic leukemia.[23] The leukemia cells of up to 80 percent of infants with ALL-1 have a chromosomal rearrangement that fuses the MLL1 gene to a gene on a different chromosome. | ||
Revision as of 19:45, 28 April 2020
Histone-lysine N-methyltransferase 2A KMT2A
| |||||||||||