Vm24 Scorpion Toxin
From Proteopedia
| Line 14: | Line 14: | ||
Vm24 is a member of the short scorpion toxin superfamily[https://www.uniprot.org/uniprot/?query=family%3A%22short+scorpion+toxin+superfamily%22&sort=score], potassium channel inhibitor family[https://www.uniprot.org/uniprot/?query=family:%22short+scorpion+toxin+superfamily.+Potassium+channel+inhibitor+family%22&sort=score]. Vm24 appears as a sister clade to the α-KTx subfamilies 6 and 7, and is thus proposed as a novel new subfamily α-KTx 23.1[https://www.uniprot.org/uniprot/?query=family:%22short+scorpion+toxin+superfamily.+Potassium+channel+inhibitor+family.+Alpha-KTx+23+subfamily%22&sort=score]. The alpha-KTx 23 subfamily contains proteins with the CSalpha/beta fold, which comprises one or two short alpha helices connected to anti-parallel beta-sheets stabilized by three or four disulfide bonds. | Vm24 is a member of the short scorpion toxin superfamily[https://www.uniprot.org/uniprot/?query=family%3A%22short+scorpion+toxin+superfamily%22&sort=score], potassium channel inhibitor family[https://www.uniprot.org/uniprot/?query=family:%22short+scorpion+toxin+superfamily.+Potassium+channel+inhibitor+family%22&sort=score]. Vm24 appears as a sister clade to the α-KTx subfamilies 6 and 7, and is thus proposed as a novel new subfamily α-KTx 23.1[https://www.uniprot.org/uniprot/?query=family:%22short+scorpion+toxin+superfamily.+Potassium+channel+inhibitor+family.+Alpha-KTx+23+subfamily%22&sort=score]. The alpha-KTx 23 subfamily contains proteins with the CSalpha/beta fold, which comprises one or two short alpha helices connected to anti-parallel beta-sheets stabilized by three or four disulfide bonds. | ||
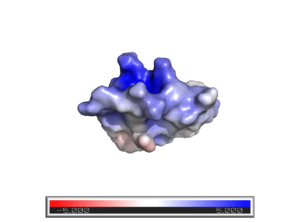
==Structural Functionality== | ==Structural Functionality== | ||
| + | [[Image:Screen Shot 2020-04-28 at 3.59.13 PM]] | ||
Revision as of 20:00, 28 April 2020
Contents |
Introduction
Vm24 synthetic scorpion toxin is a peptide toxin isolated from Vaejovis mexicanus smithi[1] scorpion venom. It is a potent inhibitor of Kv1.3 potassium channels of human T lymphocytes. Its high affinity and specificity for human lymphocytes makes it a candidate for the treatment of several autoimmune disorders.
|
Protein Source
Gurolla G.B et al [2] isolated the peptide components of Vaejovis mexicanus smithi venom using high-performance liquid chromatography. Over 200 components were identified. The structure of Vm24 was determined by solution NMR spectroscopy, and it was sequenced via trypsin digestion. The a synthetic Vm24 gene was created and artificially translated to produce the synthetic Vm24 toxin displayed. The synthetic peptide has the same mass, elution time, and physiological effects as the natural peptide.
General Structure
Vm24 is a single chain protein consisting of 36 amino acids. It has a molecular weight of 3,873 daltons. It consists of a three strand (S1,S2,S3) anti-parallel and one single turn with holding the chain together. The disulfides exist between , , , and . The disulfide bonds between and with the C-terminal strand of the β-sheet. The disulfide bridge between connects the The abundant disulfides in this protein are highly important in conferring it's unusual, strained conformation [3]. Positions and are not well defined because they have a reduced number of Nuclear Overhauser effects NOEs [4] All and are buried entirely, but all other residues are exposed. and are in a very flexible region, which is interesting because they are adjacent to a .
Function
Vm24 is a potent and selective inhibitor of Kv1.3 potassium channels of human T lymphocytes. In vitro, Kv1.3 current is reduced by approximately 2/3 rds in the presence of 3pM of Vm24. The synthetic and natural forms of this protein have nearly identical activity, but the synthetic toxin could be slightly more potent. Kd synthetic =0.5pM and Kd native = 2.9 pM, but this difference is mostly attributed to measurement difficulties at low concentrations [5]. At a higher concentration of 100pM, both the synthetic and native peptides reduce Kv1.3 current by 90%.
Phylogeny
Vm24 is a member of the short scorpion toxin superfamily[6], potassium channel inhibitor family[7]. Vm24 appears as a sister clade to the α-KTx subfamilies 6 and 7, and is thus proposed as a novel new subfamily α-KTx 23.1[8]. The alpha-KTx 23 subfamily contains proteins with the CSalpha/beta fold, which comprises one or two short alpha helices connected to anti-parallel beta-sheets stabilized by three or four disulfide bonds.
