User:Chris Garcia/Titin Z1Z2 complex
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
The giant protein titin, also known as connectin, is a giant protein about 1 micrometer in length that acts as a molecular spring which holds individual sarcomeres together in muscle tissue. Titin contains 320 proteins which makes it the largest human protein. It is half the length of a sarcomere with the four regions, I-band, A-band, M-line, and Z-line. The A-band is the largest section with a conserved sequence. Ig domains and fibronectin are arranged in long patterns called super repeats. The I-band is made up of only Ig domains and unique domain sequences arranged in tandem. In the M-line region lies overlapped carboxy terminus regions. The Z-line region contains the overlapped amino terminus regions. Titin is important because it provides flexibility, stability, and defines contraction speed in the muscle. Titin is composed of 300 tandem repeats of Ig domains, fibronectin repeats, and flexible coil PEVK. Isoforms of titin from insertions of single Ig-domains can be found in cardiac and skeletal muscle tissue. | The giant protein titin, also known as connectin, is a giant protein about 1 micrometer in length that acts as a molecular spring which holds individual sarcomeres together in muscle tissue. Titin contains 320 proteins which makes it the largest human protein. It is half the length of a sarcomere with the four regions, I-band, A-band, M-line, and Z-line. The A-band is the largest section with a conserved sequence. Ig domains and fibronectin are arranged in long patterns called super repeats. The I-band is made up of only Ig domains and unique domain sequences arranged in tandem. In the M-line region lies overlapped carboxy terminus regions. The Z-line region contains the overlapped amino terminus regions. Titin is important because it provides flexibility, stability, and defines contraction speed in the muscle. Titin is composed of 300 tandem repeats of Ig domains, fibronectin repeats, and flexible coil PEVK. Isoforms of titin from insertions of single Ig-domains can be found in cardiac and skeletal muscle tissue. | ||
== Structure == | == Structure == | ||
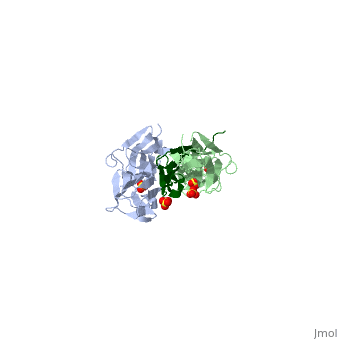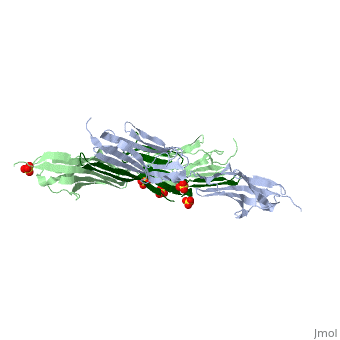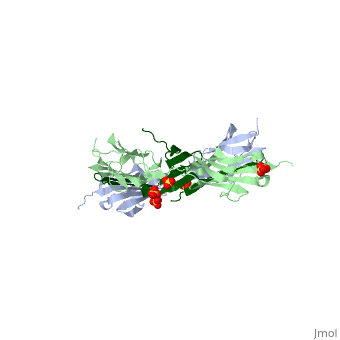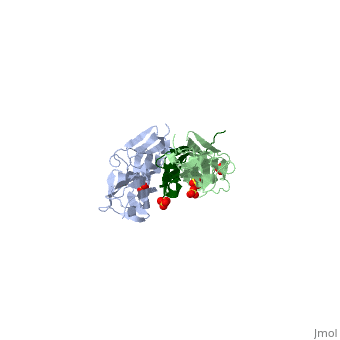
| - | Attached to the Z and M lines and half the length of the sarcomere spans the string like molecule titin. The major components of titin are β-sandwich protein modules known as immunoglobulin domains, or Ig domains. These domains contain stable cores of β-sheets which are connected by flexible loops where protrusions form binding sites for antigens. Unlike specific Ig domains involved in immunological recognition, Titin’s Ig domains contribute to the passive stiffness of a stretched sarcomere. A sarcomere’s length varies depending on the type of muscle. In cardiac muscle, sarcomere length ranges from 1.9 to 2.5 micrometers in length whereas in a skeletal calf muscle, its length ranges from 2.2 to 3.8 micrometers. Titin is built upon a linear assembly of roughly 300 immunoglobulin and fibronectin III domains with an addition to several uncharacterized domain segments. Approximately ninety percent of titin’s mass by composition | + | Attached to the Z and M lines and half the length of the sarcomere spans the string like molecule titin. The major components of titin are β-sandwich protein modules known as immunoglobulin domains, or Ig domains. These domains contain stable cores of β-sheets which are connected by flexible loops where protrusions form binding sites for antigens. Unlike specific Ig domains involved in immunological recognition, Titin’s Ig domains contribute to the passive stiffness of a stretched sarcomere. A sarcomere’s length varies depending on the type of muscle. In cardiac muscle, sarcomere length ranges from 1.9 to 2.5 micrometers in length whereas in a skeletal calf muscle, its length ranges from 2.2 to 3.8 micrometers. Titin is built upon a linear assembly of roughly 300 immunoglobulin and fibronectin III domains with an addition to several uncharacterized domain segments. Approximately ninety percent of titin’s mass by composition exist in the globular Ig or FN-III domains. Each domain consists of ∼100 residues folded into a β-sandwich motif. Two Ig domains Z1 and Z2 in complex with telethonin, a transcript in striated muscle, has revealed a novel binding motif between Ig domains and ligand. Unlike typical ligand-binding through insertion of the ligand into a receptor binding pocket, Z1Z2 domains of two antiparallel titin N-termini are joined together by an N-terminal fragment of the ligand telethonin through β-strand cross-linking.<ref> Lee, Eric H. “The Titin/Telethonin Complex.” The Titin/Telethonin Complex, 2006, www.ks.uiuc.edu/Research/telethonin/.</ref> The three-dimensional structures of five Ig domains I1, I27, Z1, Z2, and M5 each show an eight or nine stranded beta sandwich which are arranged in antiparallel fashion. The A-band or Z-line regions contain proteins located in the thin and thick filaments. The titin A-band is made from highly conserved Ig or Fibronectin III domains that are arranged in regular patterns correlating to the structural composition of associated thick filaments.<ref> Gao, Mu, and Eric Lee. “Titin Ig Domains.” Case Study: Titin Ig Domains, www.ks.uiuc.edu/Training/CaseStudies/pdfs/titin.pdf.</ref> The repeating patterns seen in both titin and myosin results in the hypothesis that the titin A-band may regulate the assembly of myosin within the early stage of muscle development. The I-band region is the only region that allows titin to move freely. The I-band region provides the passive elastic properties of the sarcomere. Titin contains variable composition in I band domains. These are called isoforms and have been found in various types of muscle tissue. This allows us to conclude that these different types of domains within the titin spring determine the stiffness and extensibility of the protein. |
== Function == | == Function == | ||
The main function of titin is to give elastic stabilization to the myosin and actin filaments. Titin has regions which mirror the different regions of the sarcomere. These regions have mechanical functions, catalytic functions, and the ability to bind to proteins. These protein molecules exhibit a role in thick and thin filament molecular scaffold assembly. The Ig domains appear to be designed to resist extreme tension. Under stress, they unfold reversibly at a higher load as the stretch in the sarcomere continues. This resistance to stress and reversible unfolding prevents our muscles from over stretching. “Titin I27 is the first structurally determined Ig domain from the I-band region responsible for regulating passive elasticity for muscle sarcomere.”<ref>Gao, Mu, and Eric Lee. “Titin Ig Domains.” Case Study: Titin Ig Domains, www.ks.uiuc.edu/Training/CaseStudies/pdfs/titin.pdf.</ref> I27 contains two beta sheets, ABED and AGFC, which are tightly connected to the terminal regions through a straightened loop are shown. | The main function of titin is to give elastic stabilization to the myosin and actin filaments. Titin has regions which mirror the different regions of the sarcomere. These regions have mechanical functions, catalytic functions, and the ability to bind to proteins. These protein molecules exhibit a role in thick and thin filament molecular scaffold assembly. The Ig domains appear to be designed to resist extreme tension. Under stress, they unfold reversibly at a higher load as the stretch in the sarcomere continues. This resistance to stress and reversible unfolding prevents our muscles from over stretching. “Titin I27 is the first structurally determined Ig domain from the I-band region responsible for regulating passive elasticity for muscle sarcomere.”<ref>Gao, Mu, and Eric Lee. “Titin Ig Domains.” Case Study: Titin Ig Domains, www.ks.uiuc.edu/Training/CaseStudies/pdfs/titin.pdf.</ref> I27 contains two beta sheets, ABED and AGFC, which are tightly connected to the terminal regions through a straightened loop are shown. | ||
Revision as of 23:56, 28 April 2020
The Titin 1ya5 with Z1Z2 domain
.
| |||||||||||