This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Adenomatous polyposis coli
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
=== Regulation of cell division === | === Regulation of cell division === | ||
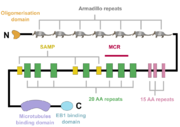
APC interacts with the plus end of microtubules and stabilises them<ref name="Mogensen2002">Mogensen, M. M. et al. (2002) ‘The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells’, Journal of Cell Biology, 157(6), pp. 1041–1048. doi: 10.1083/jcb.200203001.</ref>. During mitosis, it is involved in the regulation of spindle formation and correct chromosome attachment control<ref name="Dikovskaya2004">Dikovskaya, D., Newton, I. P. and Näthke, I. S. (2004) ‘The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts.’, Molecular biology of the cell, 15(6), pp. 2978–91. doi: 10.1091/mbc.e03-08-0613.</ref><ref name="Green2005">Green, R. A., Wollman, R. and Kaplan, K. B. (2005) ‘APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment’, Molecular Biology of the Cell, 16(10), pp. 4609–4622. doi: 10.1091/mbc.E05-03-0259.</ref>. C-terminally truncated APC is unable to participate in these interactions, which leads to defects in the mitotic spindle formation as well as in the chromosome segregation. This enhances the risk of additional mutations due to the chromosome instability<ref name="Green2003">Green, R. A. and Kaplan, K. B. (2003) ‘Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC’, Journal of Cell Biology, 163(5), pp. 949–961. doi: 10.1083/jcb.200307070.</ref><ref name="Dikovskaya2004"/><ref name="Tighe2004">Tighe, A., Johnson, V. L. and Taylor, S. S. (2004) ‘Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability’, Journal of Cell Science. The Company of Biologists Ltd, 117(26), pp. 6339–6353. doi: 10.1242/jcs.01556.</ref><ref name="Green2005"/><ref name="Dikovskaya2007">Dikovskaya, D. et al. (2007) ‘Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis’, Journal of Cell Biology, 176(2), pp. 183–195. doi: 10.1083/jcb.200610099.</ref>. | APC interacts with the plus end of microtubules and stabilises them<ref name="Mogensen2002">Mogensen, M. M. et al. (2002) ‘The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells’, Journal of Cell Biology, 157(6), pp. 1041–1048. doi: 10.1083/jcb.200203001.</ref>. During mitosis, it is involved in the regulation of spindle formation and correct chromosome attachment control<ref name="Dikovskaya2004">Dikovskaya, D., Newton, I. P. and Näthke, I. S. (2004) ‘The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts.’, Molecular biology of the cell, 15(6), pp. 2978–91. doi: 10.1091/mbc.e03-08-0613.</ref><ref name="Green2005">Green, R. A., Wollman, R. and Kaplan, K. B. (2005) ‘APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment’, Molecular Biology of the Cell, 16(10), pp. 4609–4622. doi: 10.1091/mbc.E05-03-0259.</ref>. C-terminally truncated APC is unable to participate in these interactions, which leads to defects in the mitotic spindle formation as well as in the chromosome segregation. This enhances the risk of additional mutations due to the chromosome instability<ref name="Green2003">Green, R. A. and Kaplan, K. B. (2003) ‘Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC’, Journal of Cell Biology, 163(5), pp. 949–961. doi: 10.1083/jcb.200307070.</ref><ref name="Dikovskaya2004"/><ref name="Tighe2004">Tighe, A., Johnson, V. L. and Taylor, S. S. (2004) ‘Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability’, Journal of Cell Science. The Company of Biologists Ltd, 117(26), pp. 6339–6353. doi: 10.1242/jcs.01556.</ref><ref name="Green2005"/><ref name="Dikovskaya2007">Dikovskaya, D. et al. (2007) ‘Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis’, Journal of Cell Biology, 176(2), pp. 183–195. doi: 10.1083/jcb.200610099.</ref>. | ||
| - | |||
| - | |||
=== Gain of function APC mutants === | === Gain of function APC mutants === | ||
| + | In addition to loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. | ||
== Structural insights into APC interactions == | == Structural insights into APC interactions == | ||
Revision as of 16:25, 29 April 2020
Adenomatous polyposis coli
| |||||||||||
References
- ↑ 1.0 1.1 1.2 1.3 Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.
- ↑ https://www.proteinatlas.org/ENSG00000134982-APC/tissue
- ↑ Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.
- ↑ Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.
- ↑ https://www.uniprot.org/uniprot/P25054
- ↑ 6.0 6.1 6.2 6.3 Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.
- ↑ Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.
- ↑ Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.
- ↑ Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)
- ↑ 10.0 10.1 Mitin, N. et al. (2007) ‘Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression’, Nature Structural and Molecular Biology, 14(9), pp. 814–823. doi: 10.1038/nsmb1290.
- ↑ 11.0 11.1 11.2 Kawasaki, Y., Sato, R. and Akiyama, T. (2003) ‘Mutated APC and Asef are involved in the migration of colorectal tumour cells’, Nature Cell Biology, 5(3), pp. 211–215. doi: 10.1038/ncb937.
- ↑ Kawasaki, Y. et al. (2010) ‘The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis’, Journal of Biological Chemistry, 285(2), pp. 1199–1207. doi: 10.1074/jbc.M109.040691.
- ↑ Kawasaki, Y. et al. (2009) ‘The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in ApcMin/+mice’, EMBO Reports, 10(12), pp. 1355–1362. doi: 10.1038/embor.2009.233.
- ↑ Faux, M. C. et al. (2004) ‘Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion’, Journal of Cell Science, 117(3), pp. 427–439. doi: 10.1242/jcs.00862.
- ↑ Aberle, H. et al. (1997) ‘beta-catenin is a target for the ubiquitin-proteasome pathway.’, The EMBO journal, 16(13), pp. 3797–804. doi: 10.1093/emboj/16.13.3797.
- ↑ Liu, C. et al. (2002) ‘Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism’, Cell. Cell Press, 108(6), pp. 837–847. doi: 10.1016/S0092-8674(02)00685-2.
- ↑ 17.0 17.1 Parker, T. W. and Neufeld, K. L. (2020) ‘APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes’, Scientific Reports. Nature Research, 10(1). doi: 10.1038/s41598-020-59899-z.
- ↑ 18.0 18.1 18.2 Pronobis, M. I., Rusan, N. M. and Peifer, M. (2015) ‘A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction.’, eLife. eLife Sciences Publications Ltd, 4(September 2015), p. e08022. doi: 10.7554/eLife.08022.
- ↑ 19.0 19.1 He, T. C. et al. (1998) ‘Identification of c-MYC as a target of the APC pathway’, Science, 281(5382), pp. 1509–1512. doi: 10.1126/science.281.5382.1509.
- ↑ 20.0 20.1 Tetsu, O. and McCormick, F. (1999) ‘β-catenin regulates expression of cyclin D1 in colon carcinoma cells’, Nature, 398(6726), pp. 422–426. doi: 10.1038/18884.
- ↑ Neufeld, K. L. et al. (2000) ‘APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export.’, EMBO reports, 1(6), pp. 519–23. doi: 10.1093/embo-reports/kvd117.
- ↑ Hamada, F. and Bienz, M. (2004) ‘The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF’, Developmental Cell, 7(5), pp. 677–685. doi: 10.1016/j.devcel.2004.08.022.
- ↑ Sierra, J. et al. (2006) ‘The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes.’, Genes & development, 20(5), pp. 586–600. doi: 10.1101/gad.1385806.
- ↑ Henderson, B. R. (2000) ‘Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover’, Nature Cell Biology, 2(9), pp. 653–660. doi: 10.1038/35023605.
- ↑ 25.0 25.1 Rosin-Arbesfeld, R., Townsley, F. and Blenz, M. (2000) ‘The APC tumour suppressor has a nuclear export function’, Nature, 406(6799), pp. 1009–1012. doi: 10.1038/35023016.
- ↑ Albuquerque, C. (2002) ‘The “just-right” signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade’, Human Molecular Genetics. Oxford University Press (OUP), 11(13), pp. 1549–1560. doi: 10.1093/hmg/11.13.1549.
- ↑ Schneikert, J., Grohmann, A. and Behrens, J. (2007) ‘Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner.’, Human molecular genetics, 16(2), pp. 199–209. doi: 10.1093/hmg/ddl464.
- ↑ Segditsas, S. et al. (2009) ‘APC and the three-hit hypothesis’, Oncogene, 28(1), pp. 146–155. doi: 10.1038/onc.2008.361.
- ↑ Mogensen, M. M. et al. (2002) ‘The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells’, Journal of Cell Biology, 157(6), pp. 1041–1048. doi: 10.1083/jcb.200203001.
- ↑ 30.0 30.1 Dikovskaya, D., Newton, I. P. and Näthke, I. S. (2004) ‘The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts.’, Molecular biology of the cell, 15(6), pp. 2978–91. doi: 10.1091/mbc.e03-08-0613.
- ↑ 31.0 31.1 31.2 Green, R. A., Wollman, R. and Kaplan, K. B. (2005) ‘APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment’, Molecular Biology of the Cell, 16(10), pp. 4609–4622. doi: 10.1091/mbc.E05-03-0259.
- ↑ Green, R. A. and Kaplan, K. B. (2003) ‘Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC’, Journal of Cell Biology, 163(5), pp. 949–961. doi: 10.1083/jcb.200307070.
- ↑ 33.0 33.1 Tighe, A., Johnson, V. L. and Taylor, S. S. (2004) ‘Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability’, Journal of Cell Science. The Company of Biologists Ltd, 117(26), pp. 6339–6353. doi: 10.1242/jcs.01556.
- ↑ Dikovskaya, D. et al. (2007) ‘Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis’, Journal of Cell Biology, 176(2), pp. 183–195. doi: 10.1083/jcb.200610099.
- ↑ Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.
- ↑ Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.
- ↑ Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.