Adenomatous polyposis coli
From Proteopedia
(Difference between revisions)
| Line 2: | Line 2: | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
'''<scene name='84/843011/Pokus1/1'>Adenomatous polyposis coli (APC)</scene>''' is a multidomain tumour suppressor protein involved in the regulation of various cellular processes, such as cell adhesion, migration or proliferation<ref name="Zhang2017">Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.</ref>. It is expressed in plethora of organs and tissues, e. g. cerebral cortex, bronchi or the gastrointestinal tract<ref name="proteinatlas">https://www.proteinatlas.org/ENSG00000134982-APC/tissue</ref>. Germline truncation mutations of APC result in familial adenomatous polyposis, a hereditary form of colon cancer<ref name="Ficari2000">Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.</ref>. Additionally, loss of the C-terminal portion of APC is detected in about 80 % of sporadic colon cancers<ref name="Rowan2000">Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.</ref>. | '''<scene name='84/843011/Pokus1/1'>Adenomatous polyposis coli (APC)</scene>''' is a multidomain tumour suppressor protein involved in the regulation of various cellular processes, such as cell adhesion, migration or proliferation<ref name="Zhang2017">Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.</ref>. It is expressed in plethora of organs and tissues, e. g. cerebral cortex, bronchi or the gastrointestinal tract<ref name="proteinatlas">https://www.proteinatlas.org/ENSG00000134982-APC/tissue</ref>. Germline truncation mutations of APC result in familial adenomatous polyposis, a hereditary form of colon cancer<ref name="Ficari2000">Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.</ref>. Additionally, loss of the C-terminal portion of APC is detected in about 80 % of sporadic colon cancers<ref name="Rowan2000">Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.</ref>. | ||
| + | |||
== The overall structure of APC == | == The overall structure of APC == | ||
| Line 7: | Line 8: | ||
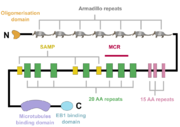
The APC protein, its primary sequence encompassing 2843 aminoacids<ref name="uniprot">https://www.uniprot.org/uniprot/P25054</ref>, consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by so called pre-ARM region and seven armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef<ref name="Zhang2012">Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.</ref>. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats<ref name="Zhang2017"/>. These motifs serve as binding sites for β-catenin<ref name="Hou2011">Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.</ref>. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin<ref name="Zhang2017"/>. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present<ref name="Su1995">Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.</ref><ref name="Zhang2017"/>. | The APC protein, its primary sequence encompassing 2843 aminoacids<ref name="uniprot">https://www.uniprot.org/uniprot/P25054</ref>, consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise. It is followed by so called pre-ARM region and seven armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef<ref name="Zhang2012">Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.</ref>. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats<ref name="Zhang2017"/>. These motifs serve as binding sites for β-catenin<ref name="Hou2011">Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.</ref>. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin<ref name="Zhang2017"/>. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present<ref name="Su1995">Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.</ref><ref name="Zhang2017"/>. | ||
Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513<ref name="Miyoshi1992">Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)</ref>. | Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513<ref name="Miyoshi1992">Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)</ref>. | ||
| + | |||
== The physiological functions of APC and their implications for colorectal cancer onset and progression == | == The physiological functions of APC and their implications for colorectal cancer onset and progression == | ||
| Line 22: | Line 24: | ||
=== Gain of function APC mutants === | === Gain of function APC mutants === | ||
In addition to loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. | In addition to loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. | ||
| + | |||
== Structural insights into APC interactions == | == Structural insights into APC interactions == | ||
| Line 28: | Line 31: | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 16:50, 29 April 2020
Adenomatous polyposis coli
| |||||||||||