We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Samantha Schneider/Sandbox1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
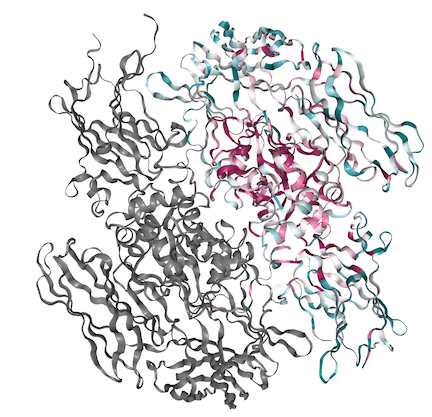
| - | The fibrin stabilization factor is a heterotetramer that circulates throughout the blood plasma as a 320 kda molecule. It consists of a dimer of A subunits and a dimer of B subunits. | + | The fibrin stabilization factor is a heterotetramer that circulates throughout the blood plasma as a 320 kda molecule. It consists of a dimer of A subunits and a dimer of B subunits. The FXIIIA subunit is composed of 4 structural units: <scene name='84/842930/Beta_sandwhich/1'>Beta sandwich</scene>, core, <scene name='84/842930/B-barrel_1/1'>barrel-1</scene>, and barrel-2 domains. The A subunit has a 37 amino acid N-terminal activation peptide, this is cleaved by thrombin during FXIII activation to FXIIIa. The [https://en.wikipedia.org/wiki/Beta-sandwich beta sandwhich] consists of residues 38-184. The <scene name='84/842930/Activation/1'>activation peptide</scene> is the first 37 amino acids on the N-terminal of the A subunit<ref>Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.</ref>. |
| - | FXIIIB subunits are glycoproteins. The B subunit is made up of ten [https://en.wikipedia.org/wiki/Sushi_domain sushi] domains | + | FXIIIB subunits are glycoproteins. The B subunit is made up of ten [https://en.wikipedia.org/wiki/Sushi_domain sushi] domains. The <scene name='84/842930/Sushi-1/1'>Sushi</scene> domains which are each composed of approximately 60 amino acids. The B subunit is known to have a protective role, but recent research has suggested that there may be a regulatory role as well. The Sushi domain's variable length loop region is shown to have a hydrophobic interaction with the N-terminal activation region of the A subunit. The variable length loop region of the sushi-1 domain is electrostatically neutral. The Cab1 site is always exposed and therefore is bound by a calcium ion<ref>Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.</ref>. |
<scene name='84/842930/Hydrophobic_tunnel/1'>Hydrophobic Tunnel</scene> is formed in the A subunit upon activation of molecule by calcium. It is the entry for the Q and K substrate to the binding site. It is formed by planar interactions of the Trp rings. The Cysteine is reactive in the binding pocket. | <scene name='84/842930/Hydrophobic_tunnel/1'>Hydrophobic Tunnel</scene> is formed in the A subunit upon activation of molecule by calcium. It is the entry for the Q and K substrate to the binding site. It is formed by planar interactions of the Trp rings. The Cysteine is reactive in the binding pocket. | ||
| Line 47: | Line 47: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | 1.Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792. | ||
Revision as of 18:05, 29 April 2020
Human Coagulation Factor XIII
| |||||||||||
References
- ↑ Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)