Adenomatous polyposis coli
From Proteopedia
| Line 12: | Line 12: | ||
=== Regulation of cell adhesion and migration === | === Regulation of cell adhesion and migration === | ||
| - | The seven armadillo repeats (ARM) together with the so-called Pre-ARM region adjoining them at the N-terminus are essential for binding the guanine nucleotide exchange factor Asef<ref name="Zhang2012"/>. In the absence of APC, Asef adopts an autoinhibited conformation, which prevents it from interaction with the small GTPase Cdc42<ref name="Mitin2007">Mitin, N. et al. (2007) ‘Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression’, Nature Structural and Molecular Biology, 14(9), pp. 814–823. doi: 10.1038/nsmb1290.</ref>. Upon APC binding, the autoinhibited conformation of Asef is disrupted and the binding site for Cdc42 is made accessible<ref name="Zhang2012"/>. Interaction with Asef leads to the exchange of GDP for GTP in the Cdc42 protein, which in turn modulates adherent junctions and contributes to enhanced cell motility<ref name="Kawasaki2003">Kawasaki, Y., Sato, R. and Akiyama, T. (2003) ‘Mutated APC and Asef are involved in the migration of colorectal tumour cells’, Nature Cell Biology, 5(3), pp. 211–215. doi: 10.1038/ncb937.</ref><ref name="Mitin2007"/><ref name="Zhang2012"/>. In colorectal cancers, the truncated version of APC with preserved Pre-ARM and ARM domains constitutively activates Asef and hence Cdc42<ref name="Kawasaki2010">Kawasaki, Y. et al. (2010) ‘The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis’, Journal of Biological Chemistry, 285(2), pp. 1199–1207. doi: 10.1074/jbc.M109.040691.</ref>. This leads to extracellular matrix remodelling and promotion of adhesion-independent growth and cell migration<ref name="Kawasaki2009">Kawasaki, Y. et al. (2009) ‘The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in ApcMin/+mice’, EMBO Reports, 10(12), pp. 1355–1362. doi: 10.1038/embor.2009.233.</ref>. | + | The seven '''armadillo repeats (ARM)''' together with the so-called '''Pre-ARM region''' adjoining them at the N-terminus are essential for binding the '''guanine nucleotide exchange factor Asef'''<ref name="Zhang2012"/>. In the absence of APC, Asef adopts an '''autoinhibited conformation''', which prevents it from interaction with the '''small GTPase Cdc42'''<ref name="Mitin2007">Mitin, N. et al. (2007) ‘Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression’, Nature Structural and Molecular Biology, 14(9), pp. 814–823. doi: 10.1038/nsmb1290.</ref>. Upon APC binding, the autoinhibited conformation of Asef is '''disrupted''' and the binding site for Cdc42 is made accessible<ref name="Zhang2012"/>. Interaction with Asef leads to the exchange of GDP for GTP in the Cdc42 protein, which in turn modulates adherent junctions and contributes to enhanced cell motility<ref name="Kawasaki2003">Kawasaki, Y., Sato, R. and Akiyama, T. (2003) ‘Mutated APC and Asef are involved in the migration of colorectal tumour cells’, Nature Cell Biology, 5(3), pp. 211–215. doi: 10.1038/ncb937.</ref><ref name="Mitin2007"/><ref name="Zhang2012"/>. In colorectal cancers, the '''truncated version of APC''' with preserved Pre-ARM and ARM domains '''constitutively activates Asef''' and hence Cdc42<ref name="Kawasaki2010">Kawasaki, Y. et al. (2010) ‘The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis’, Journal of Biological Chemistry, 285(2), pp. 1199–1207. doi: 10.1074/jbc.M109.040691.</ref>. This leads to extracellular matrix remodelling and promotion of adhesion-independent growth and cell migration<ref name="Kawasaki2009">Kawasaki, Y. et al. (2009) ‘The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in ApcMin/+mice’, EMBO Reports, 10(12), pp. 1355–1362. doi: 10.1038/embor.2009.233.</ref>. |
| - | Interestingly, APC takes part in strengthening the adherent junctions through the regulation of cellular distribution of E-cadherin and β-catenin. Full-length APC leads to increased levels of E-cadherin at the plasma membrane and decreases the pool of nuclear β-catenin in favour of the cytosolic one, enabling adherent junctions to be formed<ref name="Faux2004">Faux, M. C. et al. (2004) ‘Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion’, Journal of Cell Science, 117(3), pp. 427–439. doi: 10.1242/jcs.00862.</ref>. On the other hand, the truncated form of APC lacking the β-catenin interaction motifs is unable of such actions<ref name="Kawasaki2003"/>. | + | Interestingly, APC takes part in '''strengthening the adherent junctions''' through the regulation of cellular distribution of '''E-cadherin''' and '''β-catenin'''. Full-length APC leads to increased levels of E-cadherin at the plasma membrane and decreases the pool of nuclear β-catenin in favour of the cytosolic one, enabling adherent junctions to be formed<ref name="Faux2004">Faux, M. C. et al. (2004) ‘Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion’, Journal of Cell Science, 117(3), pp. 427–439. doi: 10.1242/jcs.00862.</ref>. On the other hand, the truncated form of APC lacking the β-catenin interaction motifs is unable of such actions<ref name="Kawasaki2003"/>. |
=== Regulation of cell proliferation through the Wnt pathway === | === Regulation of cell proliferation through the Wnt pathway === | ||
| - | APC controls cell proliferation as a negative regulator of the [https://en.wikipedia.org/wiki/Wnt_signaling_pathway Wnt signalling pathway]. Together with Axin, GSK3-β, CK1, PP2A and SCFβ-TRCP E3-ubiquitin ligase, APC forms so-called [https://en.wikipedia.org/wiki/Beta-catenin#The_beta-catenin_destruction_complex destruction complex], whose role is to promote the degradation of β-catenin<ref name="Aberle1997">Aberle, H. et al. (1997) ‘beta-catenin is a target for the ubiquitin-proteasome pathway.’, The EMBO journal, 16(13), pp. 3797–804. doi: 10.1093/emboj/16.13.3797.</ref><ref name="Liu2002">Liu, C. et al. (2002) ‘Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism’, Cell. Cell Press, 108(6), pp. 837–847. doi: 10.1016/S0092-8674(02)00685-2.</ref><ref name="Parker2020">Parker, T. W. and Neufeld, K. L. (2020) ‘APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes’, Scientific Reports. Nature Research, 10(1). doi: 10.1038/s41598-020-59899-z.</ref>. APC binds Axin through the SAMP regions in APC central part, stabilises it and enables its oligomerisation<ref name="Pronobis2015">Pronobis, M. I., Rusan, N. M. and Peifer, M. (2015) ‘A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction.’, eLife. eLife Sciences Publications Ltd, 4(September 2015), p. e08022. doi: 10.7554/eLife.08022.</ref>. Moreover, it has been proposed that APC supports the disociation of phosphorylated (=marked for degradation) β-catenin from the destruction complex<ref name="Pronobis2015"/> and that it is also essential for the movement of the destruction complex towards the plasma membrane<ref name="Parker2020"/>. However, degradation of β-catenin is not the only way employed by APC to antagonise the Wnt signalling. β-catenin binding motifs in the central part of APC compete with other interaction partners of β-catenin, such as the transcription factor TCF, thus preventing the expression of pro-proliferative factors (cyclin D, c-myc)<ref name="He1998">He, T. C. et al. (1998) ‘Identification of c-MYC as a target of the APC pathway’, Science, 281(5382), pp. 1509–1512. doi: 10.1126/science.281.5382.1509.</ref><ref name="Tetsu1999">Tetsu, O. and McCormick, F. (1999) ‘β-catenin regulates expression of cyclin D1 in colon carcinoma cells’, Nature, 398(6726), pp. 422–426. doi: 10.1038/18884.</ref><ref name="Neufeld2000">Neufeld, K. L. et al. (2000) ‘APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export.’, EMBO reports, 1(6), pp. 519–23. doi: 10.1093/embo-reports/kvd117.</ref><ref name="Hamada2004">Hamada, F. and Bienz, M. (2004) ‘The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF’, Developmental Cell, 7(5), pp. 677–685. doi: 10.1016/j.devcel.2004.08.022.</ref><ref name="Sierra2006">Sierra, J. et al. (2006) ‘The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes.’, Genes & development, 20(5), pp. 586–600. doi: 10.1101/gad.1385806.</ref>. Additionally, APC was observed to facilitate the export of β-catenin from the nucleus, hence delivering it out of reach of its target transcription factors<ref name="Henderson2000">Henderson, B. R. (2000) ‘Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover’, Nature Cell Biology, 2(9), pp. 653–660. doi: 10.1038/35023605.</ref><ref name="Rosin-Arbesfeld2000">Rosin-Arbesfeld, R., Townsley, F. and Blenz, M. (2000) ‘The APC tumour suppressor has a nuclear export function’, Nature, 406(6799), pp. 1009–1012. doi: 10.1038/35023016.</ref>. Truncated APC defective in β-catenin binding can not effectively control the Wnt pathway signalling either by promoting β-catenin degradation or by preventing it from interacting with transcription factors, which might lead to overactivation of cyclin D or c-myc expression and hence excessive proliferation<ref name="He1998"/><ref name="Tetsu1999"/><ref name="Rosin-Arbesfeld2000"/><ref name="Pronobis2015"/>. However, the first β-catenin binding repeat is often preserved in truncated APC mutants in colorectal carcinomas, which has brought forth the hypothesis that preserving some level of β-catenin downregulation is necessary to balance the potentionally harmful outcomes of constitutive β-catenin activation<ref name="Albuquerque2002">Albuquerque, C. (2002) ‘The “just-right” signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade’, Human Molecular Genetics. Oxford University Press (OUP), 11(13), pp. 1549–1560. doi: 10.1093/hmg/11.13.1549.</ref><ref name="Schneikert2007">Schneikert, J., Grohmann, A. and Behrens, J. (2007) ‘Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner.’, Human molecular genetics, 16(2), pp. 199–209. doi: 10.1093/hmg/ddl464.</ref><ref name="Segditsas2009">Segditsas, S. et al. (2009) ‘APC and the three-hit hypothesis’, Oncogene, 28(1), pp. 146–155. doi: 10.1038/onc.2008.361.</ref>. | + | APC controls cell proliferation as a '''negative regulator''' of the [https://en.wikipedia.org/wiki/Wnt_signaling_pathway Wnt signalling pathway]. Together with Axin, GSK3-β, CK1, PP2A and SCFβ-TRCP E3-ubiquitin ligase, APC forms so-called [https://en.wikipedia.org/wiki/Beta-catenin#The_beta-catenin_destruction_complex destruction complex], whose role is to promote the '''degradation of β-catenin'''<ref name="Aberle1997">Aberle, H. et al. (1997) ‘beta-catenin is a target for the ubiquitin-proteasome pathway.’, The EMBO journal, 16(13), pp. 3797–804. doi: 10.1093/emboj/16.13.3797.</ref><ref name="Liu2002">Liu, C. et al. (2002) ‘Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism’, Cell. Cell Press, 108(6), pp. 837–847. doi: 10.1016/S0092-8674(02)00685-2.</ref><ref name="Parker2020">Parker, T. W. and Neufeld, K. L. (2020) ‘APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes’, Scientific Reports. Nature Research, 10(1). doi: 10.1038/s41598-020-59899-z.</ref>. APC binds Axin through the SAMP regions in APC central part, stabilises it and enables its oligomerisation<ref name="Pronobis2015">Pronobis, M. I., Rusan, N. M. and Peifer, M. (2015) ‘A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction.’, eLife. eLife Sciences Publications Ltd, 4(September 2015), p. e08022. doi: 10.7554/eLife.08022.</ref>. Moreover, it has been proposed that APC supports the disociation of phosphorylated (=marked for degradation) β-catenin from the destruction complex<ref name="Pronobis2015"/> and that it is also essential for the movement of the destruction complex towards the plasma membrane<ref name="Parker2020"/>. However, degradation of β-catenin is not the only way employed by APC to antagonise the Wnt signalling. β-catenin binding motifs in the central part of APC compete with other interaction partners of β-catenin, such as the '''transcription factor TCF''', thus preventing the expression of '''pro-proliferative factors''' (cyclin D, c-myc)<ref name="He1998">He, T. C. et al. (1998) ‘Identification of c-MYC as a target of the APC pathway’, Science, 281(5382), pp. 1509–1512. doi: 10.1126/science.281.5382.1509.</ref><ref name="Tetsu1999">Tetsu, O. and McCormick, F. (1999) ‘β-catenin regulates expression of cyclin D1 in colon carcinoma cells’, Nature, 398(6726), pp. 422–426. doi: 10.1038/18884.</ref><ref name="Neufeld2000">Neufeld, K. L. et al. (2000) ‘APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export.’, EMBO reports, 1(6), pp. 519–23. doi: 10.1093/embo-reports/kvd117.</ref><ref name="Hamada2004">Hamada, F. and Bienz, M. (2004) ‘The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF’, Developmental Cell, 7(5), pp. 677–685. doi: 10.1016/j.devcel.2004.08.022.</ref><ref name="Sierra2006">Sierra, J. et al. (2006) ‘The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes.’, Genes & development, 20(5), pp. 586–600. doi: 10.1101/gad.1385806.</ref>. Additionally, APC was observed to facilitate the '''export of β-catenin from the nucleus''', hence delivering it out of reach of its target transcription factors<ref name="Henderson2000">Henderson, B. R. (2000) ‘Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover’, Nature Cell Biology, 2(9), pp. 653–660. doi: 10.1038/35023605.</ref><ref name="Rosin-Arbesfeld2000">Rosin-Arbesfeld, R., Townsley, F. and Blenz, M. (2000) ‘The APC tumour suppressor has a nuclear export function’, Nature, 406(6799), pp. 1009–1012. doi: 10.1038/35023016.</ref>. Truncated APC defective in β-catenin binding can not effectively control the Wnt pathway signalling either by promoting β-catenin degradation or by preventing it from interacting with transcription factors, which might lead to overactivation of cyclin D or c-myc expression and hence '''excessive proliferation'''<ref name="He1998"/><ref name="Tetsu1999"/><ref name="Rosin-Arbesfeld2000"/><ref name="Pronobis2015"/>. However, the first β-catenin binding repeat is often preserved in truncated APC mutants in colorectal carcinomas, which has brought forth the hypothesis that preserving some level of β-catenin downregulation is necessary to balance the potentionally harmful outcomes of constitutive β-catenin activation<ref name="Albuquerque2002">Albuquerque, C. (2002) ‘The “just-right” signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade’, Human Molecular Genetics. Oxford University Press (OUP), 11(13), pp. 1549–1560. doi: 10.1093/hmg/11.13.1549.</ref><ref name="Schneikert2007">Schneikert, J., Grohmann, A. and Behrens, J. (2007) ‘Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner.’, Human molecular genetics, 16(2), pp. 199–209. doi: 10.1093/hmg/ddl464.</ref><ref name="Segditsas2009">Segditsas, S. et al. (2009) ‘APC and the three-hit hypothesis’, Oncogene, 28(1), pp. 146–155. doi: 10.1038/onc.2008.361.</ref>. |
=== Regulation of cell division === | === Regulation of cell division === | ||
| - | APC interacts with the plus end of microtubules and stabilises them<ref name="Mogensen2002">Mogensen, M. M. et al. (2002) ‘The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells’, Journal of Cell Biology, 157(6), pp. 1041–1048. doi: 10.1083/jcb.200203001.</ref>. During mitosis, it is involved in the regulation of spindle formation and correct chromosome attachment control<ref name="Dikovskaya2004">Dikovskaya, D., Newton, I. P. and Näthke, I. S. (2004) ‘The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts.’, Molecular biology of the cell, 15(6), pp. 2978–91. doi: 10.1091/mbc.e03-08-0613.</ref><ref name="Green2005">Green, R. A., Wollman, R. and Kaplan, K. B. (2005) ‘APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment’, Molecular Biology of the Cell, 16(10), pp. 4609–4622. doi: 10.1091/mbc.E05-03-0259.</ref>. C-terminally truncated APC is unable to participate in these interactions, which leads to defects in the mitotic spindle formation as well as in the chromosome segregation. This enhances the risk of additional mutations due to | + | APC interacts with the '''plus end of microtubules''' and stabilises them<ref name="Mogensen2002">Mogensen, M. M. et al. (2002) ‘The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells’, Journal of Cell Biology, 157(6), pp. 1041–1048. doi: 10.1083/jcb.200203001.</ref>. During mitosis, it is involved in the regulation of spindle formation and '''correct chromosome attachment''' control<ref name="Dikovskaya2004">Dikovskaya, D., Newton, I. P. and Näthke, I. S. (2004) ‘The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts.’, Molecular biology of the cell, 15(6), pp. 2978–91. doi: 10.1091/mbc.e03-08-0613.</ref><ref name="Green2005">Green, R. A., Wollman, R. and Kaplan, K. B. (2005) ‘APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment’, Molecular Biology of the Cell, 16(10), pp. 4609–4622. doi: 10.1091/mbc.E05-03-0259.</ref>. C-terminally truncated APC is unable to participate in these interactions, which leads to '''defects in the mitotic spindle formation''' as well as in the '''chromosome segregation'''. This enhances the risk of additional mutations due to '''chromosome instability'''<ref name="Green2003">Green, R. A. and Kaplan, K. B. (2003) ‘Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC’, Journal of Cell Biology, 163(5), pp. 949–961. doi: 10.1083/jcb.200307070.</ref><ref name="Dikovskaya2004"/><ref name="Tighe2004">Tighe, A., Johnson, V. L. and Taylor, S. S. (2004) ‘Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability’, Journal of Cell Science. The Company of Biologists Ltd, 117(26), pp. 6339–6353. doi: 10.1242/jcs.01556.</ref><ref name="Green2005"/><ref name="Dikovskaya2007">Dikovskaya, D. et al. (2007) ‘Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis’, Journal of Cell Biology, 176(2), pp. 183–195. doi: 10.1083/jcb.200610099.</ref>. |
=== Gain of function APC mutants === | === Gain of function APC mutants === | ||
| - | In addition to loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. | + | In addition to the '''loss-of-function mutants''' of APC which are not able to perform its '''tumour suppressive functions''', a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a '''gain-of-function''' manner. Some studies even show that such mutant forms act in a '''dominant''' way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle<ref name="Tighe2004"/><ref name="Green2005"/>, antagonise the induction of apoptotic cell death<ref name="Qian2007">Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.</ref><ref name="Brocardo2008">Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.</ref>, enhance cell migration<ref name="Kawasaki2003"/> or compromise directional cell migration<ref name="Nelson2012">Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.</ref>. |
| Line 37: | Line 37: | ||
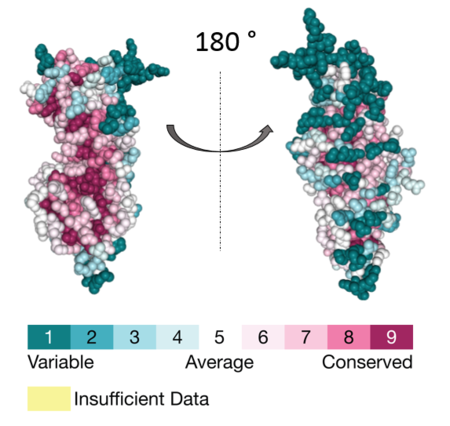
The residues lining the ABR-binding groove of APC are the most evolutionarily conserved part of APC ('''Figure 2'''), indicating that APC-Asef interaction might be a feature of their homologs as well<ref name="Zhang2012"/><ref name="Consurf1">Ben Chorin A., Masrati G., Kessel A., Narunsky A., Sprinzak J., Lahav S., Ashkenazy H. and Ben-Tal N. (2020)ConSurf-DB: An accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Science 29:258–267.</ref><ref name="Consurf2">Goldenberg O., Erez E., Nimrod G. and Ben-Tal N. (2009). | The residues lining the ABR-binding groove of APC are the most evolutionarily conserved part of APC ('''Figure 2'''), indicating that APC-Asef interaction might be a feature of their homologs as well<ref name="Zhang2012"/><ref name="Consurf1">Ben Chorin A., Masrati G., Kessel A., Narunsky A., Sprinzak J., Lahav S., Ashkenazy H. and Ben-Tal N. (2020)ConSurf-DB: An accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Science 29:258–267.</ref><ref name="Consurf2">Goldenberg O., Erez E., Nimrod G. and Ben-Tal N. (2009). | ||
The ConSurf-DB: Pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Research (Database issue), 37:D323-D327; PMID: 18971256</ref>. | The ConSurf-DB: Pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Research (Database issue), 37:D323-D327; PMID: 18971256</ref>. | ||
| - | [[Image:APC cons OK.PNG|thumb|upright= | + | [[Image:APC cons OK.PNG|thumb|upright=2.5|'''Figure 2: Conservation scores of the individual residues mapped at the surface of APC. ''' Created using the ConSurf database (https://consurfdb.tau.ac.il/)]] |
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 19:56, 30 April 2020
Contents |
Adenomatous polyposis coli
Adenomatous polyposis coli (APC) is a multidomain tumour suppressor protein involved in the regulation of various cellular processes, such as cell adhesion, migration or proliferation[1]. It is expressed in plethora of organs and tissues, e. g. cerebral cortex, bronchi or the gastrointestinal tract[2]. Germline truncation mutations of APC result in familial adenomatous polyposis, a hereditary form of colon cancer[3]. Additionally, loss of the C-terminal portion of APC is detected in about 80 % of sporadic colon cancers[4].
The overall structure of APC
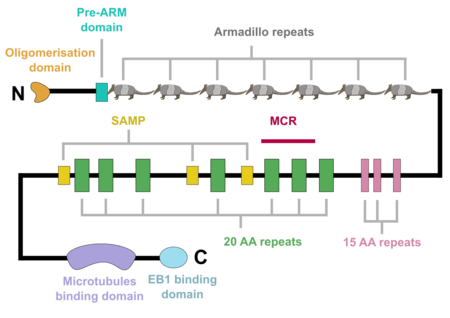
The APC protein, its primary sequence encompassing 2843 aminoacids[5], consists of multiple domains, which enable it to interact with diverse partners. At the N-terminus, an oligomerisation domain is found, enabling the APC protein to oligomerise (Figure 1). It is followed by so called Pre-ARM region and seven armadillo repeats, which form a groove for binding of a guanine nucleotide exchange factor Asef[6]. The central part of APC contains three 15 aminoacid long repeats followed by seven 20 aminoacid long repeats[1]. These motifs serve as binding sites for β-catenin[7]. In between the 20 aminoacid repeats, three SAMP regions are dispersed, enabling the interaction with Axin[1]. At the C-terminus, a basic domain responsible for binding to microtubules as well as EB1 interaction domain are present[8][1]. Interestingly, majority of somatic mutations occurs in so called mutation cluster region (MCR) between codons 1286 and 1513[9].
The physiological functions of APC and their implications for colorectal cancer onset and progression
Regulation of cell adhesion and migration
The seven armadillo repeats (ARM) together with the so-called Pre-ARM region adjoining them at the N-terminus are essential for binding the guanine nucleotide exchange factor Asef[6]. In the absence of APC, Asef adopts an autoinhibited conformation, which prevents it from interaction with the small GTPase Cdc42[10]. Upon APC binding, the autoinhibited conformation of Asef is disrupted and the binding site for Cdc42 is made accessible[6]. Interaction with Asef leads to the exchange of GDP for GTP in the Cdc42 protein, which in turn modulates adherent junctions and contributes to enhanced cell motility[11][10][6]. In colorectal cancers, the truncated version of APC with preserved Pre-ARM and ARM domains constitutively activates Asef and hence Cdc42[12]. This leads to extracellular matrix remodelling and promotion of adhesion-independent growth and cell migration[13]. Interestingly, APC takes part in strengthening the adherent junctions through the regulation of cellular distribution of E-cadherin and β-catenin. Full-length APC leads to increased levels of E-cadherin at the plasma membrane and decreases the pool of nuclear β-catenin in favour of the cytosolic one, enabling adherent junctions to be formed[14]. On the other hand, the truncated form of APC lacking the β-catenin interaction motifs is unable of such actions[11].
Regulation of cell proliferation through the Wnt pathway
APC controls cell proliferation as a negative regulator of the Wnt signalling pathway. Together with Axin, GSK3-β, CK1, PP2A and SCFβ-TRCP E3-ubiquitin ligase, APC forms so-called destruction complex, whose role is to promote the degradation of β-catenin[15][16][17]. APC binds Axin through the SAMP regions in APC central part, stabilises it and enables its oligomerisation[18]. Moreover, it has been proposed that APC supports the disociation of phosphorylated (=marked for degradation) β-catenin from the destruction complex[18] and that it is also essential for the movement of the destruction complex towards the plasma membrane[17]. However, degradation of β-catenin is not the only way employed by APC to antagonise the Wnt signalling. β-catenin binding motifs in the central part of APC compete with other interaction partners of β-catenin, such as the transcription factor TCF, thus preventing the expression of pro-proliferative factors (cyclin D, c-myc)[19][20][21][22][23]. Additionally, APC was observed to facilitate the export of β-catenin from the nucleus, hence delivering it out of reach of its target transcription factors[24][25]. Truncated APC defective in β-catenin binding can not effectively control the Wnt pathway signalling either by promoting β-catenin degradation or by preventing it from interacting with transcription factors, which might lead to overactivation of cyclin D or c-myc expression and hence excessive proliferation[19][20][25][18]. However, the first β-catenin binding repeat is often preserved in truncated APC mutants in colorectal carcinomas, which has brought forth the hypothesis that preserving some level of β-catenin downregulation is necessary to balance the potentionally harmful outcomes of constitutive β-catenin activation[26][27][28].
Regulation of cell division
APC interacts with the plus end of microtubules and stabilises them[29]. During mitosis, it is involved in the regulation of spindle formation and correct chromosome attachment control[30][31]. C-terminally truncated APC is unable to participate in these interactions, which leads to defects in the mitotic spindle formation as well as in the chromosome segregation. This enhances the risk of additional mutations due to chromosome instability[32][30][33][31][34].
Gain of function APC mutants
In addition to the loss-of-function mutants of APC which are not able to perform its tumour suppressive functions, a growing amount of experiments supports the hypothesis that the C-terminally truncated APC mutants behave in a gain-of-function manner. Some studies even show that such mutant forms act in a dominant way, e. g. actively prevent cell cycle arrest upon incorrect chromosome attachment to the mitotic spindle[33][31], antagonise the induction of apoptotic cell death[35][36], enhance cell migration[11] or compromise directional cell migration[37].
Structural insights into APC interactions
Activation of Asef
|
In the absence of APC, Asef adopts an , its four domains forming a compact structure. The , containing a binding site for Cdc42 GTPase, is tied to the through the , which makes extensive contacts with both of them. More specifically, the are involved in interaction with the DH domain and the form contacts with the PH domain[10]. In such a conformation, the Cdc42 GTPase binding site is obstructed by the [10].
The binding occurs through of APC and the . Notably, the on the surface of APC formed by . The interaction is stabilised by hydrogen-bonding network as well as by van der Waals contacts[6].
The residues lining the ABR-binding groove of APC are the most evolutionarily conserved part of APC (Figure 2), indicating that APC-Asef interaction might be a feature of their homologs as well[6][38][39].
References
- ↑ 1.0 1.1 1.2 1.3 Zhang, L. and Shay, J. W. (2017) ‘Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.’, Journal of the National Cancer Institute, 109(8). doi: 10.1093/jnci/djw332.
- ↑ https://www.proteinatlas.org/ENSG00000134982-APC/tissue
- ↑ Ficari, F. et al. (2000) ‘APC gene mutations and colorectal adenomatosis in familial adenomatous polyposis’, British Journal of Cancer. Churchill Livingstone, 82(2), pp. 348–353. doi: 10.1054/bjoc.1999.0925.
- ↑ Rowan, A. J. et al. (2000) ‘APC mutations in sporadic colorectal tumors: A mutational “hotspot” and interdependence of the “two hits”’, Proceedings of the National Academy of Sciences of the United States of America. National Academy of Sciences, 97(7), pp. 3352–3357. doi: 10.1073/pnas.97.7.3352.
- ↑ https://www.uniprot.org/uniprot/P25054
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Zhang, Z. et al. (2012) ‘Structural basis for the recognition of Asef by adenomatous polyposis coli’, Cell Research. Nature Publishing Group, 22(2), pp. 372–386. doi: 10.1038/cr.2011.119.
- ↑ Hou, F. et al. (2011) ‘MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response.’, Cell. Elsevier, 146(3), pp. 448–61. doi: 10.1016/j.cell.2011.06.041.
- ↑ Su, L. K. et al. (1995) ‘APC Binds to the Novel Protein EB’, Cancer Research, 55(14), pp. 2972–2977.
- ↑ Miyoshi, Y. et al. (1992) Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene | Human Molecular Genetics | Oxford Academic, Human Molecular Genetics, Vol. 1, No. 4 229-233. Available at: https://academic.oup.com/hmg/article/1/4/229/730109 (Accessed: 22 April 2020).)
- ↑ 10.0 10.1 10.2 10.3 Mitin, N. et al. (2007) ‘Release of autoinhibition of ASEF by APC leads to CDC42 activation and tumor suppression’, Nature Structural and Molecular Biology, 14(9), pp. 814–823. doi: 10.1038/nsmb1290.
- ↑ 11.0 11.1 11.2 Kawasaki, Y., Sato, R. and Akiyama, T. (2003) ‘Mutated APC and Asef are involved in the migration of colorectal tumour cells’, Nature Cell Biology, 5(3), pp. 211–215. doi: 10.1038/ncb937.
- ↑ Kawasaki, Y. et al. (2010) ‘The adenomatous polyposis coli-associated guanine nucleotide exchange factor Asef is involved in angiogenesis’, Journal of Biological Chemistry, 285(2), pp. 1199–1207. doi: 10.1074/jbc.M109.040691.
- ↑ Kawasaki, Y. et al. (2009) ‘The adenomatous polyposis coli-associated exchange factors Asef and Asef2 are required for adenoma formation in ApcMin/+mice’, EMBO Reports, 10(12), pp. 1355–1362. doi: 10.1038/embor.2009.233.
- ↑ Faux, M. C. et al. (2004) ‘Restoration of full-length adenomatous polyposis coli (APC) protein in a colon cancer cell line enhances cell adhesion’, Journal of Cell Science, 117(3), pp. 427–439. doi: 10.1242/jcs.00862.
- ↑ Aberle, H. et al. (1997) ‘beta-catenin is a target for the ubiquitin-proteasome pathway.’, The EMBO journal, 16(13), pp. 3797–804. doi: 10.1093/emboj/16.13.3797.
- ↑ Liu, C. et al. (2002) ‘Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism’, Cell. Cell Press, 108(6), pp. 837–847. doi: 10.1016/S0092-8674(02)00685-2.
- ↑ 17.0 17.1 Parker, T. W. and Neufeld, K. L. (2020) ‘APC controls Wnt-induced β-catenin destruction complex recruitment in human colonocytes’, Scientific Reports. Nature Research, 10(1). doi: 10.1038/s41598-020-59899-z.
- ↑ 18.0 18.1 18.2 Pronobis, M. I., Rusan, N. M. and Peifer, M. (2015) ‘A novel GSK3-regulated APC:Axin interaction regulates Wnt signaling by driving a catalytic cycle of efficient βcatenin destruction.’, eLife. eLife Sciences Publications Ltd, 4(September 2015), p. e08022. doi: 10.7554/eLife.08022.
- ↑ 19.0 19.1 He, T. C. et al. (1998) ‘Identification of c-MYC as a target of the APC pathway’, Science, 281(5382), pp. 1509–1512. doi: 10.1126/science.281.5382.1509.
- ↑ 20.0 20.1 Tetsu, O. and McCormick, F. (1999) ‘β-catenin regulates expression of cyclin D1 in colon carcinoma cells’, Nature, 398(6726), pp. 422–426. doi: 10.1038/18884.
- ↑ Neufeld, K. L. et al. (2000) ‘APC-mediated downregulation of beta-catenin activity involves nuclear sequestration and nuclear export.’, EMBO reports, 1(6), pp. 519–23. doi: 10.1093/embo-reports/kvd117.
- ↑ Hamada, F. and Bienz, M. (2004) ‘The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF’, Developmental Cell, 7(5), pp. 677–685. doi: 10.1016/j.devcel.2004.08.022.
- ↑ Sierra, J. et al. (2006) ‘The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes.’, Genes & development, 20(5), pp. 586–600. doi: 10.1101/gad.1385806.
- ↑ Henderson, B. R. (2000) ‘Nuclear-cytoplasmic shuttling of APC regulates β-catenin subcellular localization and turnover’, Nature Cell Biology, 2(9), pp. 653–660. doi: 10.1038/35023605.
- ↑ 25.0 25.1 Rosin-Arbesfeld, R., Townsley, F. and Blenz, M. (2000) ‘The APC tumour suppressor has a nuclear export function’, Nature, 406(6799), pp. 1009–1012. doi: 10.1038/35023016.
- ↑ Albuquerque, C. (2002) ‘The “just-right” signaling model: APC somatic mutations are selected based on a specific level of activation of the beta-catenin signaling cascade’, Human Molecular Genetics. Oxford University Press (OUP), 11(13), pp. 1549–1560. doi: 10.1093/hmg/11.13.1549.
- ↑ Schneikert, J., Grohmann, A. and Behrens, J. (2007) ‘Truncated APC regulates the transcriptional activity of beta-catenin in a cell cycle dependent manner.’, Human molecular genetics, 16(2), pp. 199–209. doi: 10.1093/hmg/ddl464.
- ↑ Segditsas, S. et al. (2009) ‘APC and the three-hit hypothesis’, Oncogene, 28(1), pp. 146–155. doi: 10.1038/onc.2008.361.
- ↑ Mogensen, M. M. et al. (2002) ‘The adenomatous polyposis coli protein unambiguously localizes to microtubule plus ends and is involved in establishing parallel arrays of microtubule bundles in highly polarized epithelial cells’, Journal of Cell Biology, 157(6), pp. 1041–1048. doi: 10.1083/jcb.200203001.
- ↑ 30.0 30.1 Dikovskaya, D., Newton, I. P. and Näthke, I. S. (2004) ‘The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts.’, Molecular biology of the cell, 15(6), pp. 2978–91. doi: 10.1091/mbc.e03-08-0613.
- ↑ 31.0 31.1 31.2 Green, R. A., Wollman, R. and Kaplan, K. B. (2005) ‘APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment’, Molecular Biology of the Cell, 16(10), pp. 4609–4622. doi: 10.1091/mbc.E05-03-0259.
- ↑ Green, R. A. and Kaplan, K. B. (2003) ‘Chromosome instability in colorectal tumor cells is associated with defects in microtubule plus-end attachments caused by a dominant mutation in APC’, Journal of Cell Biology, 163(5), pp. 949–961. doi: 10.1083/jcb.200307070.
- ↑ 33.0 33.1 Tighe, A., Johnson, V. L. and Taylor, S. S. (2004) ‘Truncating APC mutations have dominant effects on proliferation, spindle checkpoint control, survival and chromosome stability’, Journal of Cell Science. The Company of Biologists Ltd, 117(26), pp. 6339–6353. doi: 10.1242/jcs.01556.
- ↑ Dikovskaya, D. et al. (2007) ‘Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis’, Journal of Cell Biology, 176(2), pp. 183–195. doi: 10.1083/jcb.200610099.
- ↑ Qian, J. et al. (2007) ‘Caspase cleavage of the APC tumor suppressor and release of an amino-terminal domain is required for the transcription-independent function of APC in apoptosis’, Oncogene, 26(33), pp. 4872–4876. doi: 10.1038/sj.onc.1210265.
- ↑ Brocardo, M. et al. (2008) ‘Mitochondrial targeting of adenomatous polyposis coli protein is stimulated by truncating cancer mutations: Regulation of Bcl-2 and implications for cell survival’, Journal of Biological Chemistry, 283(9), pp. 5950–5959. doi: 10.1074/jbc.M708775200.
- ↑ Nelson, S. A. et al. (2012) ‘Tumorigenic fragments of APC cause dominant defects in directional cell migration in multiple model systems’, DMM Disease Models and Mechanisms, 5(6), pp. 940–947. doi: 10.1242/dmm.008607.
- ↑ Ben Chorin A., Masrati G., Kessel A., Narunsky A., Sprinzak J., Lahav S., Ashkenazy H. and Ben-Tal N. (2020)ConSurf-DB: An accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Science 29:258–267.
- ↑ Goldenberg O., Erez E., Nimrod G. and Ben-Tal N. (2009). The ConSurf-DB: Pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Research (Database issue), 37:D323-D327; PMID: 18971256