User:R. Jeremy Johnson/ABCG2 Transporter
From Proteopedia
(Difference between revisions)
| Line 30: | Line 30: | ||
==Disease== | ==Disease== | ||
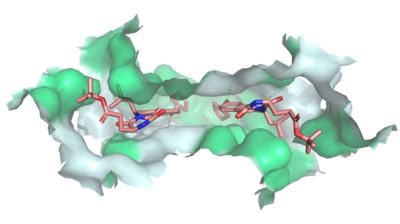
| - | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure | + | [[Image:Ligand_Interactions_6ffc.png|400 px|right|thumb|Figure 3: MZ29 bound to cavity 1 of ABCG2 [https://www.rcsb.org/structure/6FFC (6FFC)]. Two MZ29 are shown in sticks and are colored by element. Hydrophobic interactions between the surface of cavity 1 and MZ29 are shown in green.]] |
Dysfunctions in ABCG2 are linked to [https://en.wikipedia.org/wiki/Hyperuricemia hyperuricemia] which can lead to [https://en.wikipedia.org/wiki/Gout gout], [https://en.wikipedia.org/wiki/Kidney_disease kidney disease], and [https://en.wikipedia.org/wiki/Hypertension hypertension], all of which are thought to be the result of impaired transport of uric acid. Additionally, the expression of ABCG2 has been found to correlate with a poor prognosis and treatment outcome of various cancers including breast, ovarian, and lung.<ref name="Jackson"/> | Dysfunctions in ABCG2 are linked to [https://en.wikipedia.org/wiki/Hyperuricemia hyperuricemia] which can lead to [https://en.wikipedia.org/wiki/Gout gout], [https://en.wikipedia.org/wiki/Kidney_disease kidney disease], and [https://en.wikipedia.org/wiki/Hypertension hypertension], all of which are thought to be the result of impaired transport of uric acid. Additionally, the expression of ABCG2 has been found to correlate with a poor prognosis and treatment outcome of various cancers including breast, ovarian, and lung.<ref name="Jackson"/> | ||
| Line 36: | Line 36: | ||
ABCG2 hinders cancer treatment by contributing to [https://en.wikipedia.org/wiki/Multiple_drug_resistance multidrug resistance] in tumor cells. ABCG2 exports xenbiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. Cancer patients often show high levels of expression of multiple ABC transporters. For example, [https://en.wikipedia.org/wiki/Acute_myeloid_leukemia acute myeloid leukemia] (AML) has an increased expression of [https://en.wikipedia.org/wiki/P-glycoprotein ABCB1], [https://en.wikipedia.org/wiki/ABCG1 ABCG1], and ABCG2 while childhood AML shows an increased expression in [https://en.wikipedia.org/wiki/ABCA3 ABCA3], ABCB1, [https://en.wikipedia.org/wiki/ABCC3 ABCC3], and ABCG2.<ref name="Marzac">PMID:21606172</ref><ref name="Bartholomae">PMID:26512967</ref> Additionally, pancreatic cancer has shown an upregulation of [https://en.wikipedia.org/wiki/ABCB4 ABCB4], [https://en.wikipedia.org/wiki/ABCB11 ABCB11], [https://en.wikipedia.org/wiki/ABCC1 ABCC1], ABCC3, [https://en.wikipedia.org/wiki/ABCC5 ABCC5], [https://en.wikipedia.org/wiki/ABCC10 ABCC10], and ABCG2.<ref name="Mohelnikova-Duchonova">PMID:23462326</ref> | ABCG2 hinders cancer treatment by contributing to [https://en.wikipedia.org/wiki/Multiple_drug_resistance multidrug resistance] in tumor cells. ABCG2 exports xenbiotics, including vital anti-cancer drugs, which results in the inability to treat cancer cells. Cancer patients often show high levels of expression of multiple ABC transporters. For example, [https://en.wikipedia.org/wiki/Acute_myeloid_leukemia acute myeloid leukemia] (AML) has an increased expression of [https://en.wikipedia.org/wiki/P-glycoprotein ABCB1], [https://en.wikipedia.org/wiki/ABCG1 ABCG1], and ABCG2 while childhood AML shows an increased expression in [https://en.wikipedia.org/wiki/ABCA3 ABCA3], ABCB1, [https://en.wikipedia.org/wiki/ABCC3 ABCC3], and ABCG2.<ref name="Marzac">PMID:21606172</ref><ref name="Bartholomae">PMID:26512967</ref> Additionally, pancreatic cancer has shown an upregulation of [https://en.wikipedia.org/wiki/ABCB4 ABCB4], [https://en.wikipedia.org/wiki/ABCB11 ABCB11], [https://en.wikipedia.org/wiki/ABCC1 ABCC1], ABCC3, [https://en.wikipedia.org/wiki/ABCC5 ABCC5], [https://en.wikipedia.org/wiki/ABCC10 ABCC10], and ABCG2.<ref name="Mohelnikova-Duchonova">PMID:23462326</ref> | ||
| - | The substrate specificity among ABC transporters varies, so this protein family can collectively export a wide variety of substrates and, ultimately, a wide variety of anticancer drugs. ABCG2 has been known to export anticancer drugs such [https://en.wikipedia.org/wiki/Methotrexate methotrexate], [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone], [https://en.wikipedia.org/wiki/Topotecan topotecan], [https://en.wikipedia.org/wiki/Irinotecan irinotecan], and [https://en.wikipedia.org/wiki/Alvocidib flavopiridol]<ref name="Mao">PMID:25236865</ref>. Due to the high expression of multiple ABC transporters in cancer cells, simultaneous treatment of multiple transporters would likely be necessary for successful cancer treatment. | + | The substrate specificity among ABC transporters varies, so this protein family can collectively export a wide variety of substrates and, ultimately, a wide variety of anticancer drugs (Figures 3 and 4). ABCG2 has been known to export anticancer drugs such [https://en.wikipedia.org/wiki/Methotrexate methotrexate], [https://en.wikipedia.org/wiki/Mitoxantrone mitoxantrone], [https://en.wikipedia.org/wiki/Topotecan topotecan], [https://en.wikipedia.org/wiki/Irinotecan irinotecan], and [https://en.wikipedia.org/wiki/Alvocidib flavopiridol]<ref name="Mao">PMID:25236865</ref>. Due to the high expression of multiple ABC transporters in cancer cells, simultaneous treatment of multiple transporters would likely be necessary for successful cancer treatment. |
===Inhibitors=== | ===Inhibitors=== | ||
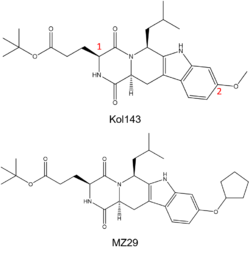
| - | [[Image:kol143 and mz29 abcg2 inhibitors.png|250 px|right|thumb|Figure | + | [[Image:kol143 and mz29 abcg2 inhibitors.png|250 px|right|thumb|Figure 4. (Top) Known ABCG2 Inhibitor Kol143; Red numbers indicate points of alternation in derivatives of inhibitor. (Bottom) Derivative of Kol143, MZ29.]] |
Due to the potential for ABCG2 inhibition to aid in cancer treatment, efforts have been made to develop specific inhibitors of ABCG2 and other ABC transporters. The ABC transporter ABCB1, also known as multidrug resistance 1 (MDR1), was a therapeutic target in previous studies which produced three generations of MDR1 inhibitors, such as [https://en.wikipedia.org/wiki/Verapamil verapamil], [https://en.wikipedia.org/wiki/Valspodar valspodar], and [https://en.wikipedia.org/wiki/Zosuquidar zosuquidar]; however, many of these inhibitors had neurotoxic effects that discouraged their use in cancer treatment.<ref name="Leonard">PMID:14530494</ref><ref name="Binkhathlan">PMID:23369096</ref><ref name="Witherspoon">PMID:9816083</ref> | Due to the potential for ABCG2 inhibition to aid in cancer treatment, efforts have been made to develop specific inhibitors of ABCG2 and other ABC transporters. The ABC transporter ABCB1, also known as multidrug resistance 1 (MDR1), was a therapeutic target in previous studies which produced three generations of MDR1 inhibitors, such as [https://en.wikipedia.org/wiki/Verapamil verapamil], [https://en.wikipedia.org/wiki/Valspodar valspodar], and [https://en.wikipedia.org/wiki/Zosuquidar zosuquidar]; however, many of these inhibitors had neurotoxic effects that discouraged their use in cancer treatment.<ref name="Leonard">PMID:14530494</ref><ref name="Binkhathlan">PMID:23369096</ref><ref name="Witherspoon">PMID:9816083</ref> | ||
| - | While ABC transporter inhibition was dismissed after failed clinical trials, interest in revisiting ABC inhibition has reemerged due to new developments made in recent years.<ref name="Robey"/> For instance, Kol143 (Figure | + | While ABC transporter inhibition was dismissed after failed clinical trials, interest in revisiting ABC inhibition has reemerged due to new developments made in recent years.<ref name="Robey"/> For instance, Kol143 (Figure 4) is a compound derived from fungal toxin [https://en.wikipedia.org/wiki/Fumitremorgin fumitremorgin C] (FTC), a selective inhibitor of ABCG2 which exhibits undesirable neurotoxic effects.<ref name="Allen">PMID:12477054</ref> Kol143 was found to be less toxic and more potent than FTC; however, this inhibitor is nonselective toward ABCG2.<ref name="Weidner">PMID:26148857</ref> Various inhibitors were derived from Kol143 with changes made at positions 1 and 2 in Figure 3, which are carbons 3 and 9 respectively. Modifications at these positions prove to affect the inhibitory capacities of compounds. A promising compound which has shown a high degree of potency is the inhibitor MZ29 (Figures 3 and 4).<ref name="Jackson"/> |
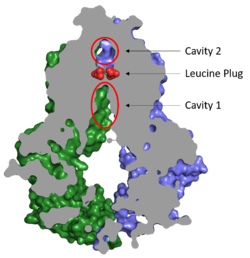
| - | ABCG2 inhibitors, <scene name='83/832932/Inhibitor_bound_cavity_1/2'>such as MZ29</scene>, bind Cavity 1 and act as competitive inhibitors against ABCG2 substrates and show a higher affinity toward the transporter. Depending on the size of the inhibitor, one or two molecules can accommodate binding to the cavity and form <scene name='83/832932/Inhibitor_interactions_cavity1/3'>hydrogen bonds, van der Waals, and stacking interactions</scene> within the binding site.<ref name="Jackson"/> Many inhibitors are too big to be transported via the leucine plug resulting in the "clogging" of the transporter. With inhibitors acting as wedges, ABCG2 is locked in the inward-facing conformation and unable to transport molecules out of the cell.<ref name="Manolaridis"/> | + | ABCG2 inhibitors, <scene name='83/832932/Inhibitor_bound_cavity_1/2'>such as MZ29</scene> (Figure 3), bind Cavity 1 and act as competitive inhibitors against ABCG2 substrates and show a higher affinity toward the transporter. Depending on the size of the inhibitor, one or two molecules can accommodate binding to the cavity and form <scene name='83/832932/Inhibitor_interactions_cavity1/3'>hydrogen bonds, van der Waals, and stacking interactions</scene> within the binding site.<ref name="Jackson"/> Many inhibitors are too big to be transported via the leucine plug resulting in the "clogging" of the transporter. With inhibitors acting as wedges, ABCG2 is locked in the inward-facing conformation and unable to transport molecules out of the cell.<ref name="Manolaridis"/> |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
Revision as of 14:14, 5 May 2020
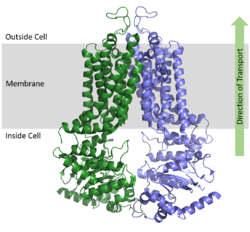
ABCG2 Multidrug Transporter
References
- ↑ 1.0 1.1 1.2 1.3 Taylor NMI, Manolaridis I, Jackson SM, Kowal J, Stahlberg H, Locher KP. Structure of the human multidrug transporter ABCG2. Nature. 2017 Jun 22;546(7659):504-509. doi: 10.1038/nature22345. Epub 2017 May, 29. PMID:28554189 doi:http://dx.doi.org/10.1038/nature22345
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Manolaridis I, Jackson SM, Taylor NMI, Kowal J, Stahlberg H, Locher KP. Cryo-EM structures of a human ABCG2 mutant trapped in ATP-bound and substrate-bound states. Nature. 2018 Nov;563(7731):426-430. doi: 10.1038/s41586-018-0680-3. Epub 2018 Nov, 7. PMID:30405239 doi:http://dx.doi.org/10.1038/s41586-018-0680-3
- ↑ 3.0 3.1 3.2 Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018 Jul;18(7):452-464. doi: 10.1038/s41568-018-0005-8. PMID:29643473 doi:http://dx.doi.org/10.1038/s41568-018-0005-8
- ↑ 4.0 4.1 4.2 4.3 Jackson SM, Manolaridis I, Kowal J, Zechner M, Taylor NMI, Bause M, Bauer S, Bartholomaeus R, Bernhardt G, Koenig B, Buschauer A, Stahlberg H, Altmann KH, Locher KP. Structural basis of small-molecule inhibition of human multidrug transporter ABCG2. Nat Struct Mol Biol. 2018 Apr;25(4):333-340. doi: 10.1038/s41594-018-0049-1. Epub, 2018 Apr 2. PMID:29610494 doi:http://dx.doi.org/10.1038/s41594-018-0049-1
- ↑ Marzac C, Garrido E, Tang R, Fava F, Hirsch P, De Benedictis C, Corre E, Lapusan S, Lallemand JY, Marie JP, Jacquet E, Legrand O. ATP Binding Cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica. 2011 Sep;96(9):1293-301. doi: 10.3324/haematol.2010.031823. Epub, 2011 May 23. PMID:21606172 doi:http://dx.doi.org/10.3324/haematol.2010.031823
- ↑ Bartholomae S, Gruhn B, Debatin KM, Zimmermann M, Creutzig U, Reinhardt D, Steinbach D. Coexpression of Multiple ABC-Transporters is Strongly Associated with Treatment Response in Childhood Acute Myeloid Leukemia. Pediatr Blood Cancer. 2016 Feb;63(2):242-7. doi: 10.1002/pbc.25785. Epub 2015 Oct, 29. PMID:26512967 doi:http://dx.doi.org/10.1002/pbc.25785
- ↑ Mohelnikova-Duchonova B, Brynychova V, Oliverius M, Honsova E, Kala Z, Muckova K, Soucek P. Differences in transcript levels of ABC transporters between pancreatic adenocarcinoma and nonneoplastic tissues. Pancreas. 2013 May;42(4):707-16. doi: 10.1097/MPA.0b013e318279b861. PMID:23462326 doi:http://dx.doi.org/10.1097/MPA.0b013e318279b861
- ↑ Mao Q, Unadkat JD. Role of the breast cancer resistance protein (BCRP/ABCG2) in drug transport--an update. AAPS J. 2015 Jan;17(1):65-82. doi: 10.1208/s12248-014-9668-6. Epub 2014 Sep 19. PMID:25236865 doi:http://dx.doi.org/10.1208/s12248-014-9668-6
- ↑ Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. Oncologist. 2003;8(5):411-24. doi: 10.1634/theoncologist.8-5-411. PMID:14530494 doi:http://dx.doi.org/10.1634/theoncologist.8-5-411
- ↑ Binkhathlan Z, Lavasanifar A. P-glycoprotein inhibition as a therapeutic approach for overcoming multidrug resistance in cancer: current status and future perspectives. Curr Cancer Drug Targets. 2013 Mar;13(3):326-46. doi:, 10.2174/15680096113139990076. PMID:23369096 doi:http://dx.doi.org/10.2174/15680096113139990076
- ↑ Witherspoon SM, Emerson DL, Kerr BM, Lloyd TL, Dalton WS, Wissel PS. Flow cytometric assay of modulation of P-glycoprotein function in whole blood by the multidrug resistance inhibitor GG918. Clin Cancer Res. 1996 Jan;2(1):7-12. PMID:9816083
- ↑ Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, Reid G, Schellens JH, Koomen GJ, Schinkel AH. Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 2002 Apr;1(6):417-25. PMID:12477054
- ↑ Weidner LD, Zoghbi SS, Lu S, Shukla S, Ambudkar SV, Pike VW, Mulder J, Gottesman MM, Innis RB, Hall MD. The Inhibitor Ko143 Is Not Specific for ABCG2. J Pharmacol Exp Ther. 2015 Sep;354(3):384-93. doi: 10.1124/jpet.115.225482. Epub , 2015 Jul 6. PMID:26148857 doi:http://dx.doi.org/10.1124/jpet.115.225482
Student Contributors
Julia Pomeroy Shelby Skaggs Sam Sullivan Jaelyn Voyles