We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:R. Jeremy Johnson/Mitochondrial Calcium Uniporter
From Proteopedia
(Difference between revisions)
| Line 4: | Line 4: | ||
== Overview == | == Overview == | ||
| - | The mitochondrial calcium uniporter (MCU) complex is the main source of entry for [https://en.wikipedia.org/wiki/Calcium calcium] ions into the [https://en.wikipedia.org/wiki/Mitochondrial_matrix mitochondrial matrix] from the [https://en.wikipedia.org/wiki/Mitochondrion#Intermembrane_space intermembrane space]. MCU channels exist in most [https://en.wikipedia.org/wiki/Eukaryote eukaryotic] life, but activity is regulated differently in each [https://en.wikipedia.org/wiki/Clade clade].<ref name="Baradaran">PMID:29995857</ref> The precise identity of the MCU wasn't discovered until 2011 and was discovered using a combination of [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy NMR spectroscopy], [https://en.wikipedia.org/wiki/Transmission_electron_cryomicroscopy cryo-electron microscopy], and [https://en.wikipedia.org/wiki/X-ray_crystallography x-ray crystallography].<ref name="Woods">PMID:31869674</ref>[https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryoelectron microscopy] (Cryo-EM) was instrumental in understanding the complete structure of the MCU. Cryo-EM analysis provided a structural framework for understanding the mechanism by with the MCU functions.<ref name="Giorgi | + | The mitochondrial calcium uniporter (MCU) complex is the main source of entry for [https://en.wikipedia.org/wiki/Calcium calcium] ions into the [https://en.wikipedia.org/wiki/Mitochondrial_matrix mitochondrial matrix] from the [https://en.wikipedia.org/wiki/Mitochondrion#Intermembrane_space intermembrane space]. MCU channels exist in most [https://en.wikipedia.org/wiki/Eukaryote eukaryotic] life, but activity is regulated differently in each [https://en.wikipedia.org/wiki/Clade clade].<ref name="Baradaran">PMID:29995857</ref> The precise identity of the MCU wasn't discovered until 2011 and was discovered using a combination of [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy NMR spectroscopy], [https://en.wikipedia.org/wiki/Transmission_electron_cryomicroscopy cryo-electron microscopy], and [https://en.wikipedia.org/wiki/X-ray_crystallography x-ray crystallography].<ref name="Woods">PMID:31869674</ref>[https://en.wikipedia.org/wiki/Cryogenic_electron_microscopy Cryoelectron microscopy] (Cryo-EM) was instrumental in understanding the complete structure of the MCU. Cryo-EM analysis provided a structural framework for understanding the mechanism by with the MCU functions.<ref name="Giorgi" /> Prior modeling of the structure was difficult because it has no apparent sequence similarity to other ion channels.<ref name="Baradaran"/> However, like other ion channels, the MCU is highly selective and efficient. The MCU has the ability to only allow calcium ions into the mitochondrial matrix at a rate of 5,000,000 ions per second even though [https://en.wikipedia.org/wiki/Potassium potassium] ions are over 100,000 times more abundant in the intermembrane space.<ref name="Baradaran"/> |
Under resting conditions, the calcium concentration in the mitochondria is about the same as in the [https://en.wikipedia.org/wiki/Cytoplasm cytoplasm], but when stimulated, it can increase calcium concentration 10-20-fold.<ref name="Giorgi">PMID:30143745</ref> Mitochondria-associated ER membranes ([https://en.wikipedia.org/wiki/Mitochondria_associated_membranes MAMs]) exist between mitochondria and the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum], the two largest cellular stores of calcium, to allow for efficient transport of calcium ions.<ref name="Wang">PMID:28882140</ref> The transfer of electrons through respiratory complexes I-IV produces the energy to pump [https://en.wikipedia.org/wiki/Hydrogen_ion hydrogen ions] into the intermembrane space (IMS) and create the proton [https://en.wikipedia.org/wiki/Electrochemical_gradient electrochemical gradient] potential.<ref name="Giorgi"/> This negative electrochemical potential is the driving force that moves positively charged calcium ions into the mitochondrial matrix.<ref name="Giorgi"/> | Under resting conditions, the calcium concentration in the mitochondria is about the same as in the [https://en.wikipedia.org/wiki/Cytoplasm cytoplasm], but when stimulated, it can increase calcium concentration 10-20-fold.<ref name="Giorgi">PMID:30143745</ref> Mitochondria-associated ER membranes ([https://en.wikipedia.org/wiki/Mitochondria_associated_membranes MAMs]) exist between mitochondria and the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum], the two largest cellular stores of calcium, to allow for efficient transport of calcium ions.<ref name="Wang">PMID:28882140</ref> The transfer of electrons through respiratory complexes I-IV produces the energy to pump [https://en.wikipedia.org/wiki/Hydrogen_ion hydrogen ions] into the intermembrane space (IMS) and create the proton [https://en.wikipedia.org/wiki/Electrochemical_gradient electrochemical gradient] potential.<ref name="Giorgi"/> This negative electrochemical potential is the driving force that moves positively charged calcium ions into the mitochondrial matrix.<ref name="Giorgi"/> | ||
| Line 14: | Line 14: | ||
===Mitochondrial Calcium Uniporter Complex=== | ===Mitochondrial Calcium Uniporter Complex=== | ||
| - | The mitochondrial calcium uniporter complex exists as a large complex (around 480 kDa in humans) made up of both pore-forming and regulatory subunits.<ref name="Wang"/> The MCU is a complex composed of regulatory subunits including mitochondrial calcium uptake (MICU), essential MICU regulator (EMRE), MCU regulatory subunit b (MCUb), and MCU regulator 1 (MCUR1). <ref name="Fan | + | The mitochondrial calcium uniporter complex exists as a large complex (around 480 kDa in humans) made up of both pore-forming and regulatory subunits.<ref name="Wang"/> The MCU is a complex composed of regulatory subunits including mitochondrial calcium uptake (MICU), essential MICU regulator (EMRE), MCU regulatory subunit b (MCUb), and MCU regulator 1 (MCUR1). <ref name="Fan" /> The mitochondrial uptake proteins (MICU1 and MICU2) are regulatory proteins in the MCU complex that exist in the IMS and contain [https://en.wikipedia.org/wiki/EF_hand EF hand domains] for calcium binding to control transport through the channel of the MCU complex.<ref name="Wang"/> When calcium ion concentration in the IMS is low, MICU1 and 2 block the MCU to prevent uptake of calcium.<ref name="Wang"/> In the presence of high calcium concentrations, more calcium binds to these regulatory proteins and they undergo a conformational change to allow calcium ions through the MCU and into the matrix.<ref name="Wang"/> In fact, when calcium levels are below 500 nM, MICU1 can block movement of calcium by itself, calcium levels between 500 nM and 1,500 nM require both MICU1 and MICU2 to block ion entry, and any concentration over 1,500 nM is sufficient for calcium entry.<ref name="Giorgi"/> Another regulatory protein, MCUR1 is a cofactor in the assembly of the [https://en.wikipedia.org/wiki/Electron_transport_chain respiratory chain] rather than an essential part of the uniporter.<ref name="Giorgi"/> Though the MCU is able to take up calcium independently, there are two other pore-forming subunits, the MCUb and the essential MCU regulator (EMRE).<ref name="Wang"/> MCUb is similar to MCU, but certain amino acids differ and make it an inhibitory subunit.<ref name="Wang"/> The EMRE is located in the IMS and connects MICU1 and MICU2 to the MCU.<ref name="Giorgi"/> It also contributes to regulation of calcium intake in the MCU.<ref name="Wang"/> |
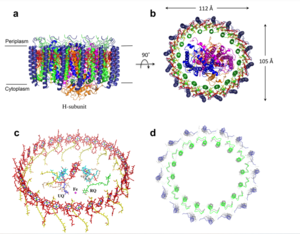
[[Image:structure.png|300 px|right|thumb|Figure 1: Structure of mitochondrial calcium uniporter colored by functional domain. The transmembrane domain is highlighted in salmon, the matrix domain in light cyan, coiled-coil domain in dark violet, and the N-terminal domain in slate blue. [https://en.wikipedia.org/wiki/Protein_Data_Bank PDB] [https://www.rcsb.org/structure/6DT0 6DT0]]] | [[Image:structure.png|300 px|right|thumb|Figure 1: Structure of mitochondrial calcium uniporter colored by functional domain. The transmembrane domain is highlighted in salmon, the matrix domain in light cyan, coiled-coil domain in dark violet, and the N-terminal domain in slate blue. [https://en.wikipedia.org/wiki/Protein_Data_Bank PDB] [https://www.rcsb.org/structure/6DT0 6DT0]]] | ||
===Mitochondrial Calcium Uniporter Structure=== | ===Mitochondrial Calcium Uniporter Structure=== | ||
| Line 24: | Line 24: | ||
===Coiled-coil domain=== | ===Coiled-coil domain=== | ||
| - | Past the transmembrane domain, the N-terminal domains of the <scene name='83/832952/Tm1/2'>TM1</scene> helices extend into the matrix and form coiled-coils with a C-terminal helix.<ref name="Baradaran"/> These "legs" are separated from each other which allows enough space for calcium ions to diffuse out into the matrix.<ref name="Baradaran"/> The <scene name='83/837230/Coiled_coil/3'>coiled coil</scene> is the first subsection of the soluble domain, which resides in the inner mitochondrial membrane. The coiled coil functions as the joints of the uniporter, providing flexibility to promote transport of Calcium ions down their concentration gradient.<ref name="Fan | + | Past the transmembrane domain, the N-terminal domains of the <scene name='83/832952/Tm1/2'>TM1</scene> helices extend into the matrix and form coiled-coils with a C-terminal helix.<ref name="Baradaran"/> These "legs" are separated from each other which allows enough space for calcium ions to diffuse out into the matrix.<ref name="Baradaran"/> The <scene name='83/837230/Coiled_coil/3'>coiled coil</scene> is the first subsection of the soluble domain, which resides in the inner mitochondrial membrane. The coiled coil functions as the joints of the uniporter, providing flexibility to promote transport of Calcium ions down their concentration gradient.<ref name="Fan" /> When calcium binds to the selectivity pore, the coiled coil swings approximately 8° around its end near the <scene name='83/837230/Nterm/2'>N-Terminal Domain</scene>. This movement propagates to the top of the transmembrane domain, where the pore is located about 85 Å away. The largest displacement triggered by the movement of the coiled coil is in the transmembrane domain, where the coil bends 20°, moving the transmembrane domain further apart. The junction between the transmembrane domain and the coiled coil's flexibility can be attributed to the disordered packing between subunits; subunits A and C adopt different conformations than the B and D subunits, although they superimpose closely.<ref name="Fan" /> The coiled-coil domain is also responsible for assembly of the MCU and is [https://en.wikipedia.org/wiki/Post-translational_modification post-translationally modified].<ref name="Fan"/> |
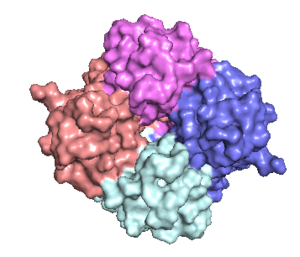
[[Image:Nterm.png|300 px|right|thumb|Figure 2: Symmetry and organization of subunits from looking down into the uniporter from the inner mitochondrial membrane [https://en.wikipedia.org/wiki/Protein_Data_Bank PDB] [https://www.rcsb.org/structure/6DT0 6DT0]]] | [[Image:Nterm.png|300 px|right|thumb|Figure 2: Symmetry and organization of subunits from looking down into the uniporter from the inner mitochondrial membrane [https://en.wikipedia.org/wiki/Protein_Data_Bank PDB] [https://www.rcsb.org/structure/6DT0 6DT0]]] | ||
===N-terminal Domain=== | ===N-terminal Domain=== | ||
Finally, each leg ends in a <scene name='83/832952/New_ones/4'>non-translated domain</scene> (NTD).<ref name="Baradaran"/> While the MCU can intake calcium without the NTD, it may have regulatory functions and the ability to bend transmembrane helices to constrict the pore.<ref name="Baradaran"/> <ref name="Fan"/> | Finally, each leg ends in a <scene name='83/832952/New_ones/4'>non-translated domain</scene> (NTD).<ref name="Baradaran"/> While the MCU can intake calcium without the NTD, it may have regulatory functions and the ability to bend transmembrane helices to constrict the pore.<ref name="Baradaran"/> <ref name="Fan"/> | ||
| - | The N-Terminal domain (NTD) is involved in calcium condition. Reorganization in the NTD due to shifts in the coiled coil switch subunits to alter membrane packing causing the interactions between the tyrosines and transmembrane helices. This propagation facilitates a rotamer switch between one pair of tyrosine controlling calcium flow through the pore. The soluble domain is wider than the transmembrane domain, allowing calcium ions to rehydrate and increasing the conductivity of ions through the uniporter into the mitochondrial matrix.<ref name="Fan | + | The N-Terminal domain (NTD) is involved in calcium condition. Reorganization in the NTD due to shifts in the coiled coil switch subunits to alter membrane packing causing the interactions between the tyrosines and transmembrane helices. This propagation facilitates a rotamer switch between one pair of tyrosine controlling calcium flow through the pore. The soluble domain is wider than the transmembrane domain, allowing calcium ions to rehydrate and increasing the conductivity of ions through the uniporter into the mitochondrial matrix.<ref name="Fan" /> |
===Selectivity Filter=== | ===Selectivity Filter=== | ||
| Line 36: | Line 36: | ||
The <scene name='83/832952/Dxxe_motif/7'>WDXXEP</scene> motif consists of <scene name='83/832952/Tryptophan/2'>Trp224</scene> at the N-terminal end, <scene name='83/832952/Selectivity_filter_asp/2'>Asp225</scene>, <scene name='83/832952/Selectivity_filter_glu/3'>Glu228</scene>, and <scene name='83/832952/New_ones/5'>Pro229</scene>.<ref name="Baradaran"/> The negatively charged side chains of Asp225 and <scene name='83/832933/Glu_358/4'>Glu228</scene> point towards the pore.<ref name="Baradaran"/> The <scene name='83/832933/Diameter/2'>diameter</scene> of the carboxyl ring is about 4Å, allowing only a dehydrated Ca<sup>2+</sup> ion to bind. The combination of these radii and high negative charge account for the selectivity of the MCU. For example, potassium has an [https://en.wikipedia.org/wiki/Ionic_radius ionic radius] of 1.38Å which is much larger than the 1.00Å ionic radius of calcium and potassium cannot fit into the negatively charged ring formed by <scene name='83/832952/Selectivity_filter_glu/3'>Glu228</scene>.<ref name="Baradaran"/> Additionally, even though sodium ions have a similar ionic radius, the +2 charge on calcium is better matched to coordination with the glutamate residues.<ref name="Baradaran"/> | The <scene name='83/832952/Dxxe_motif/7'>WDXXEP</scene> motif consists of <scene name='83/832952/Tryptophan/2'>Trp224</scene> at the N-terminal end, <scene name='83/832952/Selectivity_filter_asp/2'>Asp225</scene>, <scene name='83/832952/Selectivity_filter_glu/3'>Glu228</scene>, and <scene name='83/832952/New_ones/5'>Pro229</scene>.<ref name="Baradaran"/> The negatively charged side chains of Asp225 and <scene name='83/832933/Glu_358/4'>Glu228</scene> point towards the pore.<ref name="Baradaran"/> The <scene name='83/832933/Diameter/2'>diameter</scene> of the carboxyl ring is about 4Å, allowing only a dehydrated Ca<sup>2+</sup> ion to bind. The combination of these radii and high negative charge account for the selectivity of the MCU. For example, potassium has an [https://en.wikipedia.org/wiki/Ionic_radius ionic radius] of 1.38Å which is much larger than the 1.00Å ionic radius of calcium and potassium cannot fit into the negatively charged ring formed by <scene name='83/832952/Selectivity_filter_glu/3'>Glu228</scene>.<ref name="Baradaran"/> Additionally, even though sodium ions have a similar ionic radius, the +2 charge on calcium is better matched to coordination with the glutamate residues.<ref name="Baradaran"/> | ||
| - | scene name='83/832952/Tryptophan_proline/2'>Trp224 and Pro229</scene> pack against each other and are oriented towards the pore, but only serve to stabilize <scene name='83/832952/Selectivity_filter_glu/4'>Asp225 and Glu228</scene> and not interact with calcium ions.<ref name="Baradaran"/><ref name="Fan"/> Trp224 stabilizes the carbonyl side chains through <scene name='83/832933/H_bond_trp354_glu358/3'>hydrogen bonding</scene> and anion pi interactions. These Trp residues also form stacking interactions with Pro229, which orientate the Glu carboxyl side chains towards the middle of the pore to interact with Ca<sup>2+</sup> ions.<ref name=”Yoo”>PMID:29954988</ref> Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12Å wide pore allowing high conductivity. <ref name="Fan | + | scene name='83/832952/Tryptophan_proline/2'>Trp224 and Pro229</scene> pack against each other and are oriented towards the pore, but only serve to stabilize <scene name='83/832952/Selectivity_filter_glu/4'>Asp225 and Glu228</scene> and not interact with calcium ions.<ref name="Baradaran"/><ref name="Fan"/> Trp224 stabilizes the carbonyl side chains through <scene name='83/832933/H_bond_trp354_glu358/3'>hydrogen bonding</scene> and anion pi interactions. These Trp residues also form stacking interactions with Pro229, which orientate the Glu carboxyl side chains towards the middle of the pore to interact with Ca<sup>2+</sup> ions.<ref name=”Yoo”>PMID:29954988</ref> Approximately one helical turn below the glutamate ring of the selectivity filter, there is a tyrosine ring coming a 12Å wide pore allowing high conductivity. <ref name="Fan" /> The wider opening allows calcium to rehydrate once they pass the selectivity pore. |
[http://www.rcsb.org/structure/6DT0 Calcium Uniporter Structure] The X residues (<scene name='83/832952/New_ones/6'>Val226 and Met227</scene> in this case) face away from the pore and are exposed to the membrane.<ref name="Baradaran"/> | [http://www.rcsb.org/structure/6DT0 Calcium Uniporter Structure] The X residues (<scene name='83/832952/New_ones/6'>Val226 and Met227</scene> in this case) face away from the pore and are exposed to the membrane.<ref name="Baradaran"/> | ||
| Line 68: | Line 68: | ||
===Heart Failure=== | ===Heart Failure=== | ||
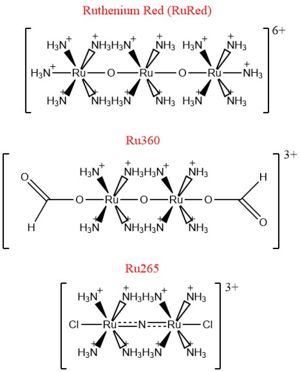
| - | Calcium overload in the mitochondria of cardiac cells lead to [https://en.wikipedia.org/wiki/Apoptosis apoptotic] cardiac cell death. Calcium governs [https://en.wikipedia.org/wiki/Cardiac_excitation-contraction_coupling excitation contraction coupling] (EC coupling) of the cardiac muscles, which creates the ATP needed to power the contraction during heart beats. The increase in mitochondrial Ca2+ concentration is essential for the functioning of this muscle contraction. Mitochondrial Ca2+ overload, though, leads to necrotic cardiac cell death and can be targeted with regulation of the MCU. An example of treatment would be with the use of Ru360 to inhibit the uptake of Ca2+ ions into the mitochondria. <ref name="Giorgi | + | Calcium overload in the mitochondria of cardiac cells lead to [https://en.wikipedia.org/wiki/Apoptosis apoptotic] cardiac cell death. Calcium governs [https://en.wikipedia.org/wiki/Cardiac_excitation-contraction_coupling excitation contraction coupling] (EC coupling) of the cardiac muscles, which creates the ATP needed to power the contraction during heart beats. The increase in mitochondrial Ca2+ concentration is essential for the functioning of this muscle contraction. Mitochondrial Ca2+ overload, though, leads to necrotic cardiac cell death and can be targeted with regulation of the MCU. An example of treatment would be with the use of Ru360 to inhibit the uptake of Ca2+ ions into the mitochondria. <ref name="Giorgi" /> |
</StructureSection> | </StructureSection> | ||
==References== | ==References== | ||
Revision as of 19:21, 8 May 2020
Mitochondrial Calcium Uniporter (MCU) Complex
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0331-8. doi:, 10.1038/s41586-018-0331-8. PMID:29995857 doi:http://dx.doi.org/10.1038/s41586-018-0331-8
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 Woods JJ, Wilson JJ. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr Opin Chem Biol. 2019 Dec 20;55:9-18. doi: 10.1016/j.cbpa.2019.11.006. PMID:31869674 doi:http://dx.doi.org/10.1016/j.cbpa.2019.11.006
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017 Sep 7;24(1):70. doi: 10.1186/s12929-017-0375-3. PMID:28882140 doi:http://dx.doi.org/10.1186/s12929-017-0375-3
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9
- ↑ Yoo J, Wu M, Yin Y, Herzik MA Jr, Lander GC, Lee SY. Cryo-EM structure of a mitochondrial calcium uniporter. Science. 2018 Jun 28. pii: science.aar4056. doi: 10.1126/science.aar4056. PMID:29954988 doi:http://dx.doi.org/10.1126/science.aar4056
Student Contributors
Ryan Heumann
Lizzy Ratz
Holly Rowe
Madi Summers
Rieser Wells