User:Tania Girao Mangolini/Sandbox 1
From Proteopedia
| Line 13: | Line 13: | ||
There are sites for N glycosylation in the loop regions, at <scene name='84/845930/Asnnglicosylation/2'>Asn13, Asn57, Asn58, Asn186, Asn198, Asn214, Asn255 and Asn268</scene> residues<ref name="review"/>. All these glycosylated Asn residues are located on the surface of C1A<ref name="ref2"/>. | There are sites for N glycosylation in the loop regions, at <scene name='84/845930/Asnnglicosylation/2'>Asn13, Asn57, Asn58, Asn186, Asn198, Asn214, Asn255 and Asn268</scene> residues<ref name="review"/>. All these glycosylated Asn residues are located on the surface of C1A<ref name="ref2"/>. | ||
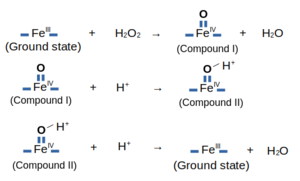
| - | Other residues play essencial roles in the the molecule, as the <scene name='84/845930/Arg38/2'>Arg38</scene> and <scene name='84/845930/Hist42/1'>His42</scene>, which are related to the formation and stabilization of <scene name='84/845930/Compoundi/1'>Compound I</scene> ([http://www.rcsb.org/structure/1HCH 1HCH]), that is the active form of this enzyme | + | Other residues play essencial roles in the the molecule, as the <scene name='84/845930/Arg38/2'>Arg38</scene> and <scene name='84/845930/Hist42/1'>His42</scene>, which are related to the formation and stabilization of <scene name='84/845930/Compoundi/1'>Compound I</scene> ([http://www.rcsb.org/structure/1HCH 1HCH]), that is the active form of this enzyme in the Peroxydase Cycle: |
| + | [[Image:Horseadish_peroxydase_cycle_v2.png|300px]] | ||
| - | + | <scene name='84/845930/Compoundi_oxy/1'>Compound I Oxygen</scene> | |
| - | [ | + | <scene name='84/845930/Compoundii_v2/1'>Compound II</scene> ([http://www.rcsb.org/structure/1H55 1H55]) |
| - | + | Besides the HRP C1A's Peroxydase Cycle, it is also known an Oxidase Cycle, that is related to defense against patogens and wounds. This cycle is responsible for the production of the produce the <scene name='84/845930/Compoundiii/1'>Compound III</scene> ([http://www.rcsb.org/structure/1H57 1H57]): | |
| - | [[Image: | + | [[Image:Horseadish_oxydase_cycle_v2.png|300px]] |
| - | <scene name='84/845930/ | + | <scene name='84/845930/Compoundiii_peroxidases/1'>Compound III Peroxidases</scene> |
| - | <scene name='84/845930/Compoundii_v2/1'>Compound II</scene> ([http://www.rcsb.org/structure/1H55 1H55]) | ||
| - | |||
| - | <scene name='84/845930/Compoundiii/1'>Compound III</scene> ([http://www.rcsb.org/structure/1H57 1H57]) | ||
| - | |||
| - | <scene name='84/845930/Compoundiii_peroxidases/1'>Compound III Peroxidases</scene> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Revision as of 03:07, 1 June 2020
HORSERADISH PEROXIDASE C1A
|
Horseadish (Armoracia rusticana) is a plant that belongs to the Brassicaceae family. The roots of this plant are rich in peroxidases, being the HRP C isozymes the most common ones. [1] However, most of the HRP research has focused on one isozyme: (1H58) [2].
Structural highlights
HRP C1A is composed by 308 residues and the residue at is Ile according to the GenBank entry M37156.1 but Tyr according to the GenBank entry HE963800.1 [2].
The molecule has a predominantly α-helical , with the exception of one short β-sheet region, and it is separated into a distal and a proximal region, each one with a .
In the center of HRP C1A there is a , which is linked to the molecule by a coordinate bond of the heme iron with a conserved residue [1].
There are sites for N glycosylation in the loop regions, at residues[1]. All these glycosylated Asn residues are located on the surface of C1A[2].
Other residues play essencial roles in the the molecule, as the and , which are related to the formation and stabilization of (1HCH), that is the active form of this enzyme in the Peroxydase Cycle:
(1H55)
Besides the HRP C1A's Peroxydase Cycle, it is also known an Oxidase Cycle, that is related to defense against patogens and wounds. This cycle is responsible for the production of the produce the (1H57):
References
- ↑ 1.0 1.1 1.2 Veitch, N.C. Horseadich peroxidase: a modern view of a classic enzyme 65:249-259 (2004). DOI: 10.1016/j.phytochem.2003.10.022
- ↑ 2.0 2.1 2.2 Krainer, F.W; GLIEDER, A. An updated view on horseradish peroxidases: recombinant production and biotechnological applications v. 99, pages 1611–1625 (2015). DOI: 10.1007/s00253-014-6346-7