User:Tania Girao Mangolini/Sandbox 1
From Proteopedia
| Line 14: | Line 14: | ||
Other residues play essencial roles in the the molecule, as the <scene name='84/845930/Arg38/2'>Arg38</scene> and <scene name='84/845930/Hist42/1'>His42</scene>, which are related to the formation and stabilization of the <scene name='84/845930/Compoundi/1'>Compound I</scene> ([http://www.rcsb.org/structure/1HCH 1HCH]), which is the product of a redox reaction between the HRP enzyme and hydrogen peroxide. | Other residues play essencial roles in the the molecule, as the <scene name='84/845930/Arg38/2'>Arg38</scene> and <scene name='84/845930/Hist42/1'>His42</scene>, which are related to the formation and stabilization of the <scene name='84/845930/Compoundi/1'>Compound I</scene> ([http://www.rcsb.org/structure/1HCH 1HCH]), which is the product of a redox reaction between the HRP enzyme and hydrogen peroxide. | ||
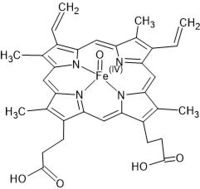
| - | In this reaction, an <scene name='84/845930/Compoundi_oxy/1'> oxygen atom | + | In this reaction, an <scene name='84/845930/Compoundi_oxy/1'> oxygen atom donated by the hydrogen peroxide binds to the heme group</scene>. The image below shows in details the heme group associated with the oxygen: |
[[Image:Horseadich_compoundI_hemeO.jpg|200px]] | [[Image:Horseadich_compoundI_hemeO.jpg|200px]] | ||
Revision as of 21:14, 2 June 2020
HORSERADISH PEROXIDASE C1A
|
Horseadish (Armoracia rusticana) is a plant that belongs to the Brassicaceae family. The roots of this plant are rich in peroxidases, being the HRP C isozymes the most common ones. [1] However, most of the HRP research has focused on one isozyme: (1H58) [2].
Structural highlights
HRP C1A is composed by 308 residues and the residue at is Ile according to the GenBank entry M37156.1 but Tyr according to the GenBank entry HE963800.1 [2].
The molecule has a predominantly α-helical , with the exception of one short β-sheet region, and it is separated into a distal and a proximal region, each one with a .
In the center of HRP C1A there is a , which is linked to the molecule by a coordinate bond of the heme iron with a conserved residue [1].
There are sites for N glycosylation in the loop regions, at residues[1]. All these glycosylated Asn residues are located on the surface of C1A[2].
Other residues play essencial roles in the the molecule, as the and , which are related to the formation and stabilization of the (1HCH), which is the product of a redox reaction between the HRP enzyme and hydrogen peroxide. In this reaction, an . The image below shows in details the heme group associated with the oxygen:
Cyclically, the Compound I forms the (1H55), which loses electrons to restore the HRP enzyme's original state:
Besides the HRP C1A's Peroxydase Cycle, it is also known an Oxidase Cycle, that is related to defense against patogens and wounds [1]. This cycle is responsible for the production of the (1H57), which has some associated to it.
References
- ↑ 1.0 1.1 1.2 1.3 Veitch, N.C. Horseadich peroxidase: a modern view of a classic enzyme 65:249-259 (2004). DOI: 10.1016/j.phytochem.2003.10.022
- ↑ 2.0 2.1 2.2 Krainer, F.W; GLIEDER, A. An updated view on horseradish peroxidases: recombinant production and biotechnological applications v. 99, pages 1611–1625 (2015). DOI: 10.1007/s00253-014-6346-7