User:Tania Girao Mangolini/Sandbox 1
From Proteopedia
| Line 13: | Line 13: | ||
There are sites for N glycosylation in the loop regions, at <scene name='84/845930/Asnnglicosylation/2'>Asn13, Asn57, Asn58, Asn186, Asn198, Asn214, Asn255 and Asn268</scene> residues<ref name="review"/>. All these glycosylated Asn residues are located on the surface of C1A<ref name="ref2"/>. | There are sites for N glycosylation in the loop regions, at <scene name='84/845930/Asnnglicosylation/2'>Asn13, Asn57, Asn58, Asn186, Asn198, Asn214, Asn255 and Asn268</scene> residues<ref name="review"/>. All these glycosylated Asn residues are located on the surface of C1A<ref name="ref2"/>. | ||
| - | Other residues play essencial roles in the the molecule, as the <scene name='84/845930/Arg38/2'>Arg38</scene> and <scene name='84/845930/Hist42/1'>His42</scene>, which are related to the formation and stabilization of the <scene name='84/845930/Compoundi/1'>Compound I</scene> ([http://www.rcsb.org/structure/1HCH 1HCH]) | + | Other residues play essencial roles in the the molecule, as the <scene name='84/845930/Arg38/2'>Arg38</scene> and <scene name='84/845930/Hist42/1'>His42</scene>, which are related to the formation and stabilization of the <scene name='84/845930/Compoundi/1'>Compound I</scene> ([http://www.rcsb.org/structure/1HCH 1HCH])in the Peroxidase Cycle. |
| - | + | ||
| - | + | == Peroxidase Cycle == | |
| - | + | Peroxidases catalyse the oxidation of a wide variety of substrates, such as phenols, aromatic amines, thioanisoles and iodide, by H2O2. The reaction is a three-step cyclic process, in which the enzyme is first oxidised by H2O2 and then reduced back to the native form in two sequential steps involving the formation of two enzyme intermediates, Compounds I and <scene name='84/845930/Compoundii_v2/1'>Compound II</scene> ([http://www.rcsb.org/structure/1H55 1H55]). | |
[[Image:Horseadish_peroxidase_cycle_v3.jpg|300px]] | [[Image:Horseadish_peroxidase_cycle_v3.jpg|300px]] | ||
| - | Besides the HRP C1A's Peroxydase Cycle, it is also known an Oxidase Cycle, that is related to defense against patogens and wounds <ref name="review"/>. This cycle is responsible for the production of the <scene name='84/845930/Compoundiii/1'>Compound III</scene> ([http://www.rcsb.org/structure/1H57 1H57]), which has some <scene name='84/845930/Compoundiii_peroxidases/1'>peroxidases</scene> associated to it. | + | == Oxidase Cycle == |
| + | |||
| + | Besides the HRP C1A's Peroxydase Cycle, it is also known an Oxidase Cycle, that is related to defense against patogens and wounds <ref name="review"/>. This cycle is responsible for the production of the <scene name='84/845930/Compoundiii/1'>Compound III</scene> ([http://www.rcsb.org/structure/1H57 1H57]), which has some <scene name='84/845930/Compoundiii_peroxidases/1'>peroxidases</scene> associated to it <ref name="ref3">Azevedo AM, Martins VC, Prazeres DM, Vojinović V, Cabral JM, Fonseca LP. Horseradish peroxidase: a valuable tool in biotechnology. Biotechnol Annu Rev. 2003;9:199‐247 [https://doi.org/10.1016/S1387-2656(03)09003-3 DOI: 10.1016/s1387-2656(03)09003-3]</ref>. | ||
[[Image:Horseadish_oxidase_cycle_v2.jpg|300px]] | [[Image:Horseadish_oxidase_cycle_v2.jpg|300px]] | ||
| Line 28: | Line 29: | ||
==Biotechnological Uses == | ==Biotechnological Uses == | ||
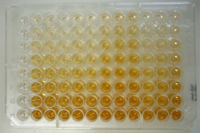
| - | HRP conjugates have been extensively used in immunoassays, such as [http://en.wikipedia.org/wiki/ELISA ELISA] (Enzyme-linked Immunosorbent Assays), [http://en.wikipedia.org/wiki/Western_blot Western-blotting] and [http://en.wikipedia.org/wiki/Immunohistochemistry Immunohistochemistry (IHC)] techniques <ref name="ref3" | + | HRP conjugates have been extensively used in immunoassays, such as [http://en.wikipedia.org/wiki/ELISA ELISA] (Enzyme-linked Immunosorbent Assays), [http://en.wikipedia.org/wiki/Western_blot Western-blotting] and [http://en.wikipedia.org/wiki/Immunohistochemistry Immunohistochemistry (IHC)] techniques <ref name="ref3"/>. |
[[Image:HRP_ELISA.jpg|200px|right|thumb|ELISA — Enzyme Linked Immuno Sorbent Assay]] | [[Image:HRP_ELISA.jpg|200px|right|thumb|ELISA — Enzyme Linked Immuno Sorbent Assay]] | ||
Revision as of 06:43, 9 June 2020
Contents |
HORSERADISH PEROXIDASE C1A
|
Horseadish (Armoracia rusticana) is a plant that belongs to the Brassicaceae family. The roots of this plant are rich in peroxidases, being the HRP C isozymes the most common ones. [1] However, most of the HRP research has focused on one isozyme: (1H58) [2].
Structural highlights
HRP C1A is composed by 308 residues and the residue at is Ile according to the GenBank entry M37156.1 but Tyr according to the GenBank entry HE963800.1 [2].
The molecule has a predominantly α-helical , with the exception of one short β-sheet region, and it is separated into a distal and a proximal region, each one with a .
In the center of HRP C1A there is a , which is linked to the molecule by a coordinate bond of the heme iron with a conserved residue [1].
There are sites for N glycosylation in the loop regions, at residues[1]. All these glycosylated Asn residues are located on the surface of C1A[2].
Other residues play essencial roles in the the molecule, as the and , which are related to the formation and stabilization of the (1HCH)in the Peroxidase Cycle.
Peroxidase Cycle
Peroxidases catalyse the oxidation of a wide variety of substrates, such as phenols, aromatic amines, thioanisoles and iodide, by H2O2. The reaction is a three-step cyclic process, in which the enzyme is first oxidised by H2O2 and then reduced back to the native form in two sequential steps involving the formation of two enzyme intermediates, Compounds I and (1H55).
Oxidase Cycle
Besides the HRP C1A's Peroxydase Cycle, it is also known an Oxidase Cycle, that is related to defense against patogens and wounds [1]. This cycle is responsible for the production of the (1H57), which has some associated to it [3].
Biotechnological Uses
HRP conjugates have been extensively used in immunoassays, such as ELISA (Enzyme-linked Immunosorbent Assays), Western-blotting and Immunohistochemistry (IHC) techniques [3].
HRP is ideal to the preparation of markers and tracers for immunoassays due to its capability of generate chromogenic products from commercially available substrates with high efficiency and to its stability enabling long term storage [3].
ELISA and Western-blotting use HRP conjugated to antibodies/antigens that bind to the desired species, this way the analyte can be visualised with a indirect HRP chromogenic essay with peroxide and a chromogenic substrate which produces an insoluble colored product [3].
Searching the thermofisher catalog is possible to find ELISA enzyme-substrate pairs, which most of them currently being HRP-based.
The application in histo- and cytochemistry is also well established, either alone as a protein tracer or conjugated with antibodies for immuno labelling. HRP is one of the most frequently used label for a large variety of molecules used in IHC techniques. In the presence of H2O2, HRP produces detectable products visible by light and electron microscopy, permitting precise cell/tissue localisation [3]. Organic Synthesis.
Enzymes are widely recognised as one of the most promissor catalysts for fine chemical synthesis. They have the ability to produce chemo, stereo and regiospecific chemicals, under mild and environmentally friendly conditions. HRP has proven to be a catalyst to a set of reactions using a green oxidant (peroxide) and to be a chiral derivatizing agent [3].
HRP have been used as a polymerization catalyst for phenols and vinyl monomers, such as acrylamides and methacrylates and for site-specific hydroxylation, such as for L-DOPA (a parkinson’s disease drug), which have been produced using HRP hydroxylation from L-tyrosine (it is not the currently industrial method for its production) and for adrenaline from L-phenylephrine [3].
References
- ↑ 1.0 1.1 1.2 1.3 Veitch, N.C. Horseadich peroxidase: a modern view of a classic enzyme 65:249-259 (2004). DOI: 10.1016/j.phytochem.2003.10.022
- ↑ 2.0 2.1 2.2 Krainer, F.W; GLIEDER, A. An updated view on horseradish peroxidases: recombinant production and biotechnological applications v. 99, pages 1611–1625 (2015). DOI: 10.1007/s00253-014-6346-7
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Azevedo AM, Martins VC, Prazeres DM, Vojinović V, Cabral JM, Fonseca LP. Horseradish peroxidase: a valuable tool in biotechnology. Biotechnol Annu Rev. 2003;9:199‐247 DOI: 10.1016/s1387-2656(03)09003-3