We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Dora Bonadio/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 7: | Line 7: | ||
== Function == | == Function == | ||
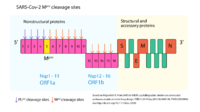
The Mpro protein function is mainly deduced from the function of SARS-CoV virus Mpro, which has a 96% amino acid identity and a highly similar three-dimensional structure with SARS-CoV-2 Mpro <ref name="Crystal_structure" />. As a [[protease]], Mpro is an enzyme that causes proteolysis, which means that it breaks protein peptide bonds by hydrolysis <ref name="fundamentals"> Sharma A, Gupta SP. Fundamentals of Viruses and Their Proteases. Viral Proteases and Their Inhibitors. 2017;1‐24. doi:[http://dx.doi.org/10.1016/B978-0-12-809712-0.00001-0]</ref>. Indeed, the Mpro processes the replicase polyprotein 1ab (pp1ab ~790 kDa) translated from the viral RNA ORF1ab <ref name="Crystal_structure" /><ref name="replication"> Enjuanes, Luis, ed. 2005. Coronavirus Replication and Reverse Genetics. Current Topics in Microbiology and Immunology. Berlin Heidelberg: Springer-Verlag. Doi:[https://doi.org/10.1007/b138038]</ref>. In fact, Mpro cleaves 11 sites of pp1ab and the recognition sequence at most sites is between Leu-Gln and (Ser, Ala, Gly) <ref name="Crystal_structure" /><ref name="sars_mers" />. Proteins resulting from this polyprotein cleavage are non-structural proteins (NSPs) and they seem to contribute with viral replication and transcription <ref name="replication" />. Thus, by processing an important number of non-structural proteins, this enzyme plays a critical role in SARS-CoV-2 replication. | The Mpro protein function is mainly deduced from the function of SARS-CoV virus Mpro, which has a 96% amino acid identity and a highly similar three-dimensional structure with SARS-CoV-2 Mpro <ref name="Crystal_structure" />. As a [[protease]], Mpro is an enzyme that causes proteolysis, which means that it breaks protein peptide bonds by hydrolysis <ref name="fundamentals"> Sharma A, Gupta SP. Fundamentals of Viruses and Their Proteases. Viral Proteases and Their Inhibitors. 2017;1‐24. doi:[http://dx.doi.org/10.1016/B978-0-12-809712-0.00001-0]</ref>. Indeed, the Mpro processes the replicase polyprotein 1ab (pp1ab ~790 kDa) translated from the viral RNA ORF1ab <ref name="Crystal_structure" /><ref name="replication"> Enjuanes, Luis, ed. 2005. Coronavirus Replication and Reverse Genetics. Current Topics in Microbiology and Immunology. Berlin Heidelberg: Springer-Verlag. Doi:[https://doi.org/10.1007/b138038]</ref>. In fact, Mpro cleaves 11 sites of pp1ab and the recognition sequence at most sites is between Leu-Gln and (Ser, Ala, Gly) <ref name="Crystal_structure" /><ref name="sars_mers" />. Proteins resulting from this polyprotein cleavage are non-structural proteins (NSPs) and they seem to contribute with viral replication and transcription <ref name="replication" />. Thus, by processing an important number of non-structural proteins, this enzyme plays a critical role in SARS-CoV-2 replication. | ||
| - | [[Image:Clivagesites sarscov2.png |thumbnail| | + | [[Image:Clivagesites sarscov2.png |thumbnail|200px|alt=A large clock tower and other buildings line a great river.|The Palace of Westminster]] |
== Structure == | == Structure == | ||
Revision as of 07:43, 11 June 2020
SARS-CoV-2 main protease (Mpro)
| |||||||||||