User:Felipe de Melo Santana/Sandbox 1
From Proteopedia
| Line 41: | Line 41: | ||
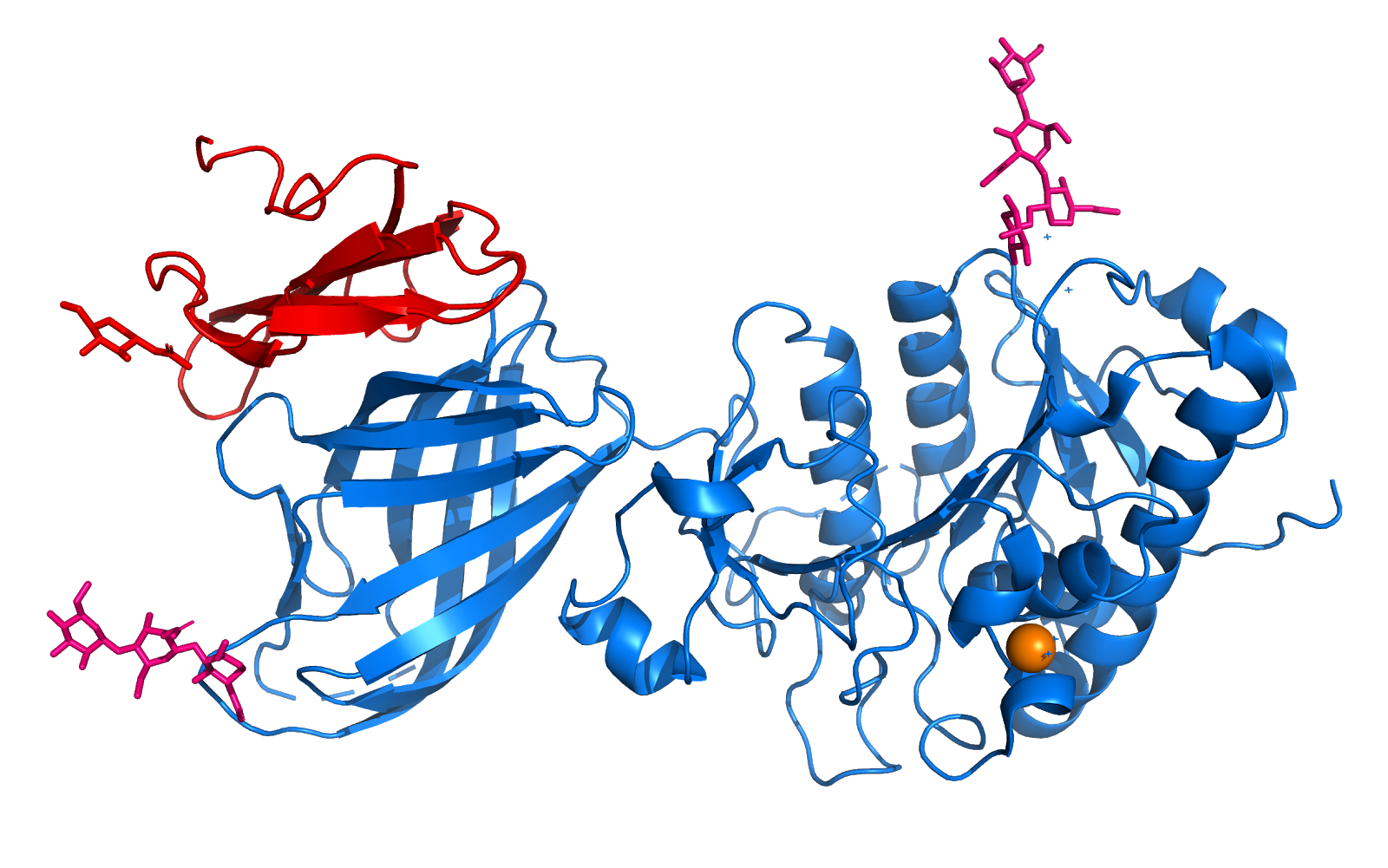
The tetramers observed in crystals of the reduced (A) and oxidized (B) forms are shown with the DNA positioned on the tetramers with plausible orientations. The tetramers are shown as ribbons (red, green, blue, and yellow for each monomer) and the model of DNA is represented as helical coils (cyan). | The tetramers observed in crystals of the reduced (A) and oxidized (B) forms are shown with the DNA positioned on the tetramers with plausible orientations. The tetramers are shown as ribbons (red, green, blue, and yellow for each monomer) and the model of DNA is represented as helical coils (cyan). | ||
| - | |||
| - | |||
| - | |||
| - | == Purification and crystallization == | ||
Revision as of 21:35, 18 June 2020
ŕ==1I69==
| |||||||||||
References
Zheng, M., Aslund, F., and Storz, G. (1998). Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721.
Choi H, Kim S, Mukhopadhyay P, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105(1):103-113. doi:10.1016/s0092-8674(01)00300-2
Toledano, M.B., Kullik, I., Trinh, F., Baird, P.T., Schneider, T.D., and Storz, G. (1994). Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78, 897–909.
Storz, G., Tartaglia, L.A., and Ames, B.N. (1990). Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248, 189–194
Kullik, I., Toledano, M.B., Tartaglia, L.A., and Storz, G. (1995a). Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J. Bacteriol. 177, 1275–1284.
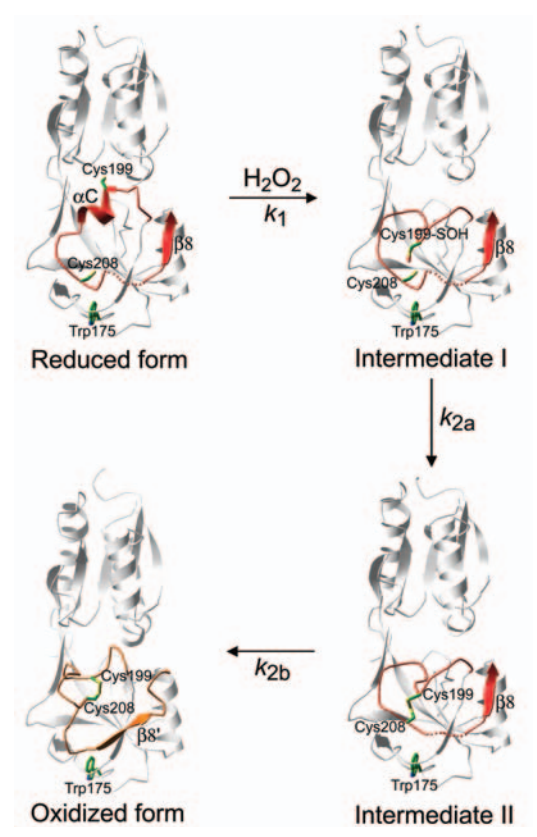
Lee C, Lee SM, Mukhopadhyay P, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11(12):1179-1185. doi:10.1038/nsmb856
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644