User:Felipe de Melo Santana/Sandbox 1
From Proteopedia
| Line 16: | Line 16: | ||
== OxyR activation == | == OxyR activation == | ||
| - | The OxyR transcription factor is activated by the formation of an intramolecular disulfide bond (Zheng et al 1998). | + | The OxyR transcription factor is activated by the formation of an intramolecular disulfide bond (Zheng et al. 1998). |
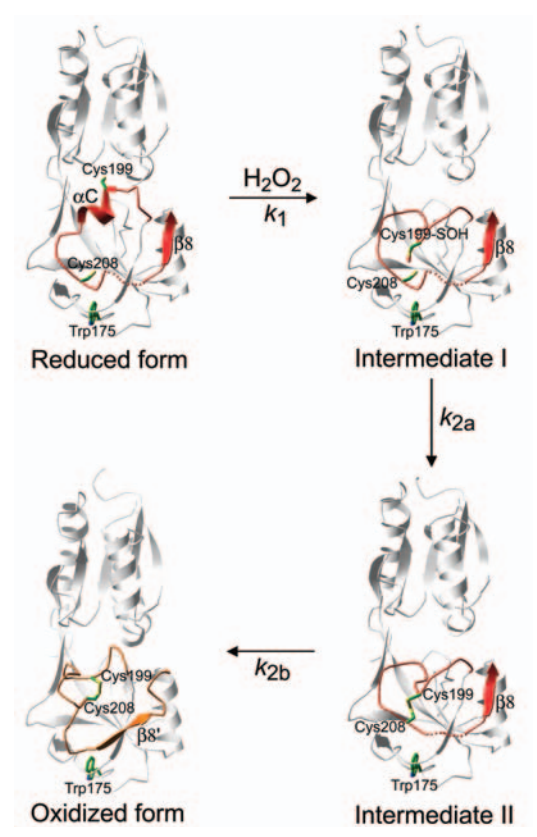
| - | The OxyR activation, by H2O2, begins with the oxidation of the <scene name='84/844928/Cys_199/1'>Cys199</scene> residue into a sulfenic acid intermediate, which causes destabilization of the Cys199 side chain in the hydrophobic binding pocket, due to its increased size and charge. The destabilization leads to expulsion of the side chain of Cys199 out of the interdomain pocket, resulting in a flexible loop around Cys199 (Lee et al 2004). | + | The OxyR activation, by H2O2, begins with the oxidation of the <scene name='84/844928/Cys_199/1'>Cys199</scene> residue into a sulfenic acid intermediate, which causes destabilization of the Cys199 side chain in the hydrophobic binding pocket, due to its increased size and charge. The destabilization leads to expulsion of the side chain of Cys199 out of the interdomain pocket, resulting in a flexible loop around Cys199 (Lee et al. 2004). |
This change in the regulatory domain of OxyR allows the formation of an intramolecular disulfide bond between Cys199 and Cys208, responsible for keeping OxyR in the active form. Thus, OxyR is deactivated by the reduction of the disulfide bond, that leads OxyR back to its reduced form (Zheng et al. 1998). | This change in the regulatory domain of OxyR allows the formation of an intramolecular disulfide bond between Cys199 and Cys208, responsible for keeping OxyR in the active form. Thus, OxyR is deactivated by the reduction of the disulfide bond, that leads OxyR back to its reduced form (Zheng et al. 1998). | ||
| Line 25: | Line 25: | ||
[[Image:Pasted_image_1.png]] | [[Image:Pasted_image_1.png]] | ||
| - | Figure 1. Intermediates in the structural transitions of OxyR. | + | Figure 1. Intermediates in the structural transitions of OxyR. Lee et al. 2004 |
== Protein activity in different forms == | == Protein activity in different forms == | ||
| Line 32: | Line 32: | ||
OxyR protein is able to DNA-binding in its two different forms, oxidized and reduced. In your oxidized form, OxyR binds to four adjacent major grooves in DNA, while in the reduced form, OxyR binds to two pairs of adjacent major grooves separated by one helical turn (Toledano et al 1994). These two modes of binding allow OxyR to regulate different promoters, under different cell conditions. | OxyR protein is able to DNA-binding in its two different forms, oxidized and reduced. In your oxidized form, OxyR binds to four adjacent major grooves in DNA, while in the reduced form, OxyR binds to two pairs of adjacent major grooves separated by one helical turn (Toledano et al 1994). These two modes of binding allow OxyR to regulate different promoters, under different cell conditions. | ||
| - | Both forms of OxyR act as repressors, regulating its own expression by oxyR transcription repression, while only oxidized OxyR is able to activate gene expression, inducing the expression of oxyS (a small regulatory RNA), hydroperoxidase I (katG), alkyl hydroperoxide reductase (ahpCF), glutathione reductase (gorA), glutaredoxin 1 (grxA) and other genes involved in redox-homeostasis, that protect the cell against oxidative stress (Zheng et al 1998). | + | Both forms of OxyR act as repressors, regulating its own expression by oxyR transcription repression, while only oxidized OxyR is able to activate gene expression, inducing the expression of oxyS (a small regulatory RNA), hydroperoxidase I (katG), alkyl hydroperoxide reductase (ahpCF), glutathione reductase (gorA), glutaredoxin 1 (grxA) and other genes involved in redox-homeostasis, that protect the cell against oxidative stress (Zheng et al. 1998). |
Revision as of 21:41, 18 June 2020
1I69
| |||||||||||
References
Zheng, M., Aslund, F., and Storz, G. (1998). Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721.
Choi H, Kim S, Mukhopadhyay P, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105(1):103-113. doi:10.1016/s0092-8674(01)00300-2
Toledano, M.B., Kullik, I., Trinh, F., Baird, P.T., Schneider, T.D., and Storz, G. (1994). Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78, 897–909.
Storz, G., Tartaglia, L.A., and Ames, B.N. (1990). Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248, 189–194
Kullik, I., Toledano, M.B., Tartaglia, L.A., and Storz, G. (1995a). Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J. Bacteriol. 177, 1275–1284.
Lee C, Lee SM, Mukhopadhyay P, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11(12):1179-1185. doi:10.1038/nsmb856
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644