User:Felipe de Melo Santana/Sandbox 1
From Proteopedia
| Line 6: | Line 6: | ||
== General information == | == General information == | ||
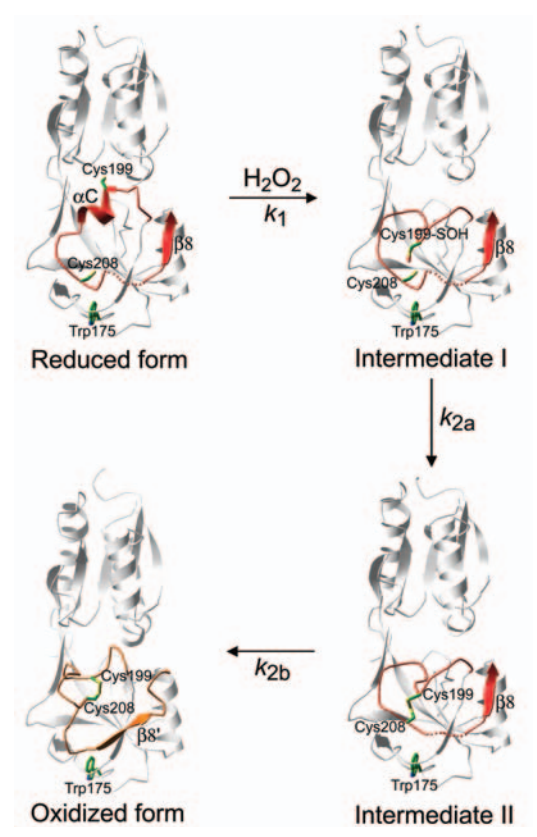
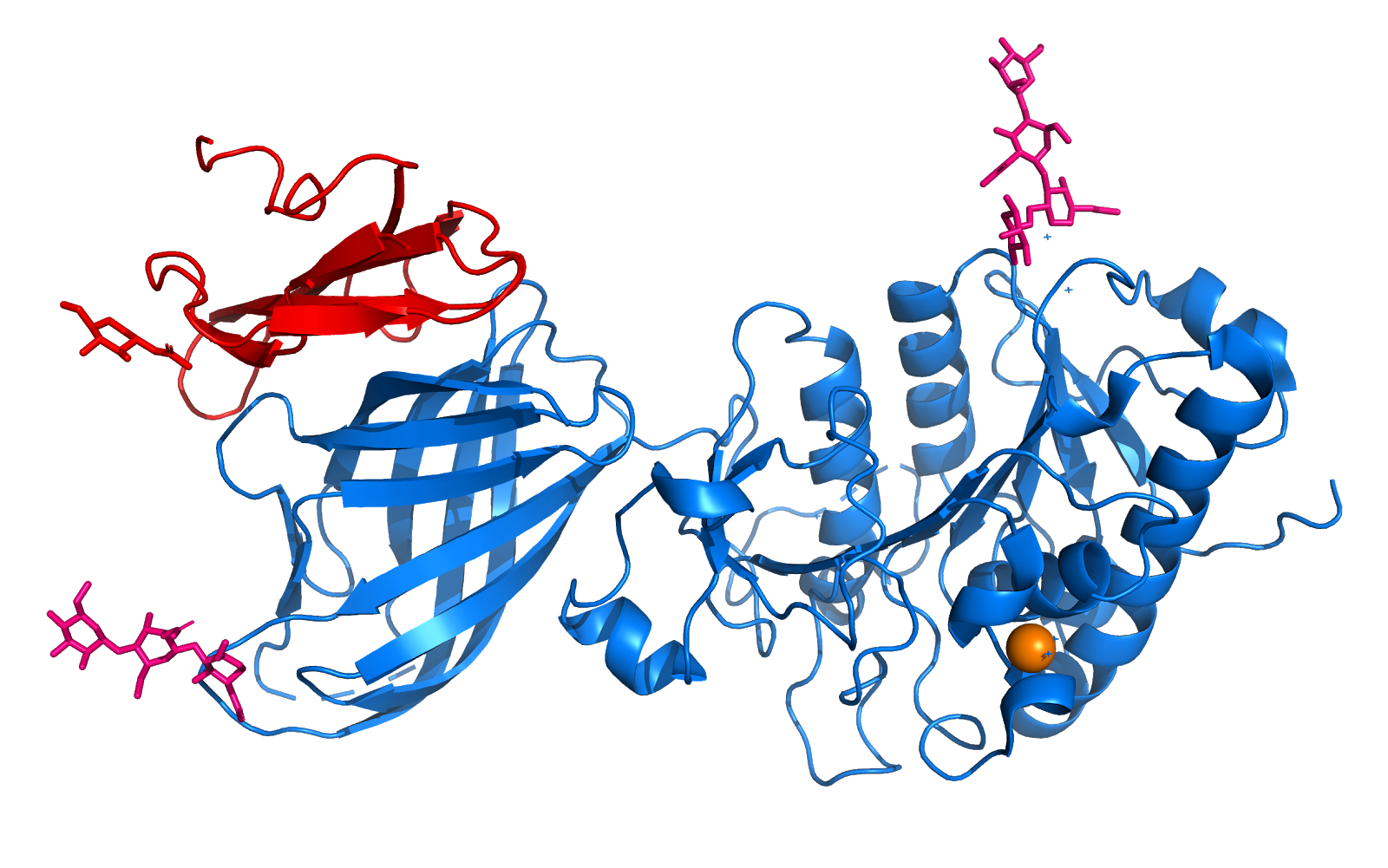
| - | OxyR is a protein that functions as a transcription factor. The OxyR we are analyzing is that of Escherichia Coli. In this perspective, the protein works by regulating the transcription of specific genes, being sensitive to H2O2.Thus, OxyR acts mainly as a transcriptional activator under oxidizing conditions through direct interaction with the RNA polymerase a subunit.This transcription factor belongs to the | + | OxyR is a protein that functions as a transcription factor. The OxyR we are analyzing is that of Escherichia Coli. In this perspective, the protein works by regulating the transcription of specific genes, being sensitive to H2O2.Thus, OxyR acts mainly as a transcriptional activator under oxidizing conditions through direct interaction with the RNA polymerase a subunit.This transcription factor belongs to the [[Lysr family]], with 34 kDa, which forms homotetramers. Under this focus, the members of this family present a domain linked to DNA in their N-terminal region, called helix-loop-helix, a central domain responsible for recognizing H2O2 that is sensitive to the regulation signal and, finally, a domain in C-terminal region responsible for activation and mutimerization. it can be concluded that the OxyR gene encodes a protein that has a dual function, acting as a stress sensor and also as a transcription activator of the OxyR operon. |
Revision as of 19:55, 20 June 2020
1I69
| |||||||||||
References
Zheng, M., Aslund, F., and Storz, G. (1998). Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721.
Choi H, Kim S, Mukhopadhyay P, et al. Structural basis of the redox switch in the OxyR transcription factor. Cell. 2001;105(1):103-113. doi:10.1016/s0092-8674(01)00300-2
Toledano, M.B., Kullik, I., Trinh, F., Baird, P.T., Schneider, T.D., and Storz, G. (1994). Redox-dependent shift of OxyR-DNA contacts along an extended DNA-binding site: a mechanism for differential promoter selection. Cell 78, 897–909.
Storz, G., Tartaglia, L.A., and Ames, B.N. (1990). Transcriptional regulator of oxidative stress-inducible genes: direct activation by oxidation. Science 248, 189–194
Kullik, I., Toledano, M.B., Tartaglia, L.A., and Storz, G. (1995a). Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J. Bacteriol. 177, 1275–1284.
Lee C, Lee SM, Mukhopadhyay P, et al. Redox regulation of OxyR requires specific disulfide bond formation involving a rapid kinetic reaction path. Nat Struct Mol Biol. 2004;11(12):1179-1185. doi:10.1038/nsmb856
Previato, M. Regulation of genes involved in oxidative stress response in Caulobacter crescentus. 2013. 134 p. Masters thesis (Microbiology) – Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo, 2013.
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644