User:Isabela Fonseca de Oliveira Granha/Sandbox 1
From Proteopedia
(Difference between revisions)
| Line 19: | Line 19: | ||
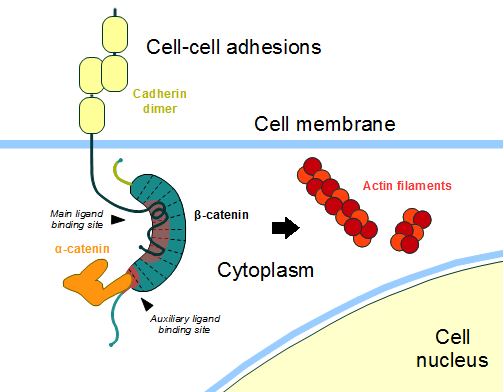
The most known interaction occurs between <scene name='84/848919/Correctbeta-catenin_e-cadherin/2'>ß-catenin (green) and E-cadherin (pink)</scene> ([http://www.rcsb.org/structure/1I7X 1I7X]) (epithelial cadherin). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the [[cadherin]]. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/1'>interaction surface</scene> is extensive, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | The most known interaction occurs between <scene name='84/848919/Correctbeta-catenin_e-cadherin/2'>ß-catenin (green) and E-cadherin (pink)</scene> ([http://www.rcsb.org/structure/1I7X 1I7X]) (epithelial cadherin). They are associated while still in the endoplasmic reticulum and interfering with the binding of these proteins results in proteasomal degradation of the [[cadherin]]. First, alpha-catenin binds to ß-catenin at the first ARM repeat, amino acids <scene name='84/848919/Corretoam118-149/1'>118-149</scene>, resulting in an alpha-catenin/ß-catenin heterodimer. This binding stabilizes ß-catenin in the hinged form, and E-cadherin can connect simultaneously. The <scene name='84/848919/Surfacebeta-catenin_e-cadherin/1'>interaction surface</scene> is extensive, covering the entire length of the ß-catenin ARM repeat domain and involving the C-terminal 100 residues of the cadherin cytoplasmic domain. <ref name="valenta2012">DOI 10.1038/emboj.2012.150</ref> <ref name="huber2001">Huber, A. H., & Weis, W. I. (2001). The structure of the β-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by β-catenin. Cell, 105(3), 391-402.</ref> | ||
| + | |||
| + | [[Image:Beta-catenin-moonlighting.png]] | ||
| + | '''Figure 1''': Adapted image of cadherin-based cell adhesion. Alpha-catenin/ß-catenin forms a heterodimer that can connects to E-cadherin promoting the adherens junctions. As a homodimer, alpha-catenin interacts with actin. | ||
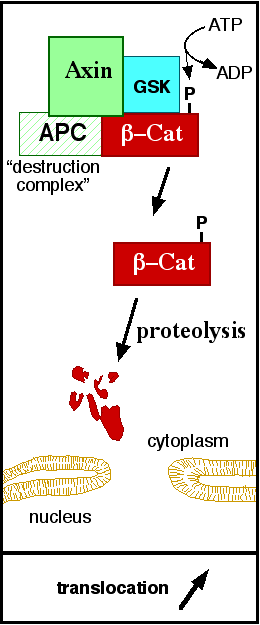
==The ß-catenin destruction complex== | ==The ß-catenin destruction complex== | ||
Revision as of 21:38, 20 June 2020
ß-catenin
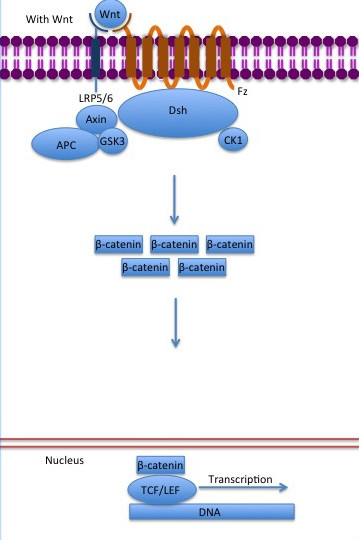
ß-catenin is an important element in cell adherens junctions connecting cytoplasmic proteins. Besides that, it is an essential regulator of the canonical Wnt pathway ([1]) (related to embryonic development). Disturbance of this activity is associated with cancer and other diseases. Therefore, ß-catenin is an important target for developing medication for many diseases, with considerable interest in its structure. [1]
| |||||||||||