User:Diogo Andrade Nani
From Proteopedia
| Line 43: | Line 43: | ||
==Eye Disorders== | ==Eye Disorders== | ||
Mutations in the SOX2 gene have been linked with several eye disorders. An example is bilateral anophthalmia, a severe structural eye deformity. This syndrome is a rare disorder characterized by abnormal development of the eyes and other parts of the body. People with SOX2 anophthalmia syndrome are usually born without eyeballs (anophthalmia), although some individuals have small eyes (microphthalmia); another related disease in this field is septo-optic dysplasia (SOD), a condition characterized by midline and forebrain abnormalities, optic nerve and pituitary hypoplasia<ref>PMID:21396578</ref>. | Mutations in the SOX2 gene have been linked with several eye disorders. An example is bilateral anophthalmia, a severe structural eye deformity. This syndrome is a rare disorder characterized by abnormal development of the eyes and other parts of the body. People with SOX2 anophthalmia syndrome are usually born without eyeballs (anophthalmia), although some individuals have small eyes (microphthalmia); another related disease in this field is septo-optic dysplasia (SOD), a condition characterized by midline and forebrain abnormalities, optic nerve and pituitary hypoplasia<ref>PMID:21396578</ref>. | ||
| - | + | ||
| - | + | ||
=References= | =References= | ||
<references /> | <references /> | ||
Revision as of 22:48, 21 June 2020
Contents |
OCT4-SOX2
Oct4 and Sox2 are two transcription factors (TFs) involved in various roles in murine and primate cells, mainly related to the maintenance of pluripotency and self-renewal properties in embryonic stem cells. These two factors, encoded by the POU5F1 (POU Class 5 Homeobox 1) and SOX2 (SRY-Box Transcription Factor 2) genes, respectively, serve as reprogramming TFs and occupy the same target genes in vivo [1][2], forming the complex OCT4-SOX2, which is the main way in which they act, although they are not obligate heterodimers in solution.
OCT4
The OCT4 transcription factor (octamer-binding transcription factor 4), also known as OCT-3, OCT3 / 4, OTF3 or NF-A3, was discovered almost three decades ago, where its use relationship with pluripotent CTE in primate and rodent species [3]. This protein is encoded by the POU5F1 gene, which is located on chromosome 6 in humans and 17 in rats, and belongs to the POU family (Pit, October, Unc) of DNA-binding proteins, which regulate the expression of target genes [3][4]. In humans, through alternative splicing, POU5F1 generates less than eight distinct RNA transcripts, these being OCT4A, OCT4B-190, OCT4B-265, OCT4B-164, OCT4B1 and more recently reported as OCT4C, OCT4C1 and OCT4B4 variants [4]. In addition to the generated isoforms, many studies have been carried out mainly with respect to the functions of the OCT4A isoform. Studies targeting the OCT4B isoforms (190, 265 and 164) that are not able to support an automatic restoration of the CTE, but they can respond to cellular stress, whereas the functions of OCT4B1, OCT4C and OCT4C1 have not yet been clarified[5]. OCT4A is normally expressed in the early stages of embryonic development and represents one of the main regulatory factors for pluripotency and self-review of embryonic stem cells, being considered a marker of pluripotency[6]. A further differentiation of CTE into cells used for different tissues depends on rapid and rapid expression of OCT4A, and these cells are differentiated remain with the OCT4A factor silenced [7][8][9]. However, we have already documented an open expression of transcription factors such as OCT4, SOX2 and NANOG, together or controlled, lead to tumors, metastases and the greatest recurrence after use, in different types of cancer [3].
SOX2
The SOX/Sox (SRY homology box) family of proteins comprises 20 individual members in man and mouse [10], which SOX2 is the most explored. SOX proteins are principally defined by a conserved DNA-binding element, the so-called high mobility group (HMG) that relates to a transcriptional master regulator of virility (i.e., SEX determining factor Y, SRY) and thus functionally qualifies SOX/Sox proteins as DNA-binders [11][12]. While Sox proteins contribute to various cellular functionalities, reprogramming capacity is largely confined to members of the SoxB1 group (i.e., Sox1, Sox2, and Sox3)[13]. SOX2 significantly often imposes transcription modulatory in conjunction with co-factors, such as Oct3/4.
The OCT4-SOX2 mechanism in the nucleosome
|
|
Transcription factors bind to DNA at specific sequene motifs. Once they're bound, the TFs are capable of regulating gene expression and govern cell identity, making it either harder or easier for RNA polymerase to bind to the promoter of the gene.
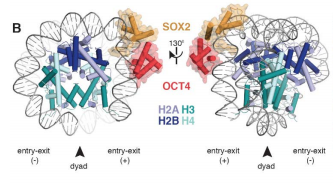
The chromatin usually restricts TFs DNA access to over 95% of nucleosomal DNA, due to the nucleosome architecture with histones H2A, H2B, H3, and H4 and its two DNA gyres. The nucleosome is the chromatin basic unit, composed of a 147 pb DNA segment wrapped around 8 histone proteins. It is a convention that the sites in which a DNA major groove is pointed to the nucleosome core are called "superhelix location" (SHL). The SHL are enumerated from 0 to ±7, having 0 as the nucleosome main axis, known as "dyad".
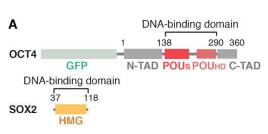
There are two possible scenarios for nucleossomal TF-engagement in this situation: TF binding without changing the nucleosomal architecture, or TF-mediated changes to the nucleosome by distorting the histone core, looping the DNA, or taking advantage of nucleosome unwrapping dynamics at the entry-exit sites[14]. The OCT4-SOX2 binds in the SHL-6 site (Fig 1)[14] and both of them act in the DNA removal from the core histones [15]. OCT4 has a bipartite DNA binding domain (DBD) comprised of a POU-specific (POUS) and POU-homeo-domain (POUHD) separated by 17-residues (Fig 2) and SOX2 has a high-mobility group (HMG) domain (Fig 2) [14] [15] [16]. The OCT4-POUS and SOX2-HMG DBDs engage major and minor grooves, respectively [15]. The DNA remains attached and straightened around the OCT4 site but is detached around the SOX2 motif [15].
OCT4 recognizes a partial motif, engaging DNA with its POUS domain, whereas the POUHD is not engaged [14]. On free DNA, both POU domains engage the major groove over 8bp on opposite sides of the DNA [15]. SOX2 competes with histones for DNA binding and kinks DNA by ~90° at SHL-6.5 away from the histones [16]. This is accomplished by intercalation of the SOX2 Phe48 and Met49 ‘wedge’ at the TT base step [16]. SOX2 kinks the DNA and synergistically with OCT4 releases the DNA from the core histones Movie1[14].
Figure 1 - OCT4-SOX2-NCPSHL+6 model[14].
Figure 2 - Domain schematic of OCT4 and SOX2 constructs[14].
Gatekeeper for embryonic stem cell pluripotency
The pluripotent identity is ruled by transcriptional factor such as Oct4 and Sox2, that act as key pluripotency regulators among the mammals [17]. Oct4 keeps the undifferentiated cells from becoming trophoblast or endoderm [17] and Sox2 is critical in the formation of pluripotent epiblast cells [18]. The forced expression of Oct4 in Sox2-null mouse embryonic stem cells can rescue the pluripotency, indicating that the role of Sox2 in maintaining the pluripotent state of embryonic stem cells is primarily to sustain a sufficient level of Oct4 expression [19] [20]. Oct4 and Sox2 cooperate to keep the pluripotency of embryonic stem cells by co-occupying a large number of enhancers and/or promoters and regulating the expression levels of their target genes [18]. They activate the transcription of genes involved in the self renewal of embryonic stem cells and besides, they bind themselves to the promoters of their own genes activating them [17].
Yamanaka reprogramming factors and Induced Pluripotend Stem Cells (iPSCs)
In 2006, Yamanaka and Takahashi first demonstrated the factors necessary for the induced Pluripotent Stem Cells (iPSC) generation. The Yamanaka factors, including Oct3/4 and Sox2 (also Klf4 and c-Myc), represent an important milestone in life sciences and have been widely used in the research and medical fields [21].
Related Diseases
Eye Disorders
Mutations in the SOX2 gene have been linked with several eye disorders. An example is bilateral anophthalmia, a severe structural eye deformity. This syndrome is a rare disorder characterized by abnormal development of the eyes and other parts of the body. People with SOX2 anophthalmia syndrome are usually born without eyeballs (anophthalmia), although some individuals have small eyes (microphthalmia); another related disease in this field is septo-optic dysplasia (SOD), a condition characterized by midline and forebrain abnormalities, optic nerve and pituitary hypoplasia[22].
References
- ↑ Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG, Gifford DK, Melton DA, Jaenisch R, Young RA. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005 Sep 23;122(6):947-56. doi: 10.1016/j.cell.2005.08.020. PMID:16153702 doi:http://dx.doi.org/10.1016/j.cell.2005.08.020
- ↑ Chen X, Xu H, Yuan P, Fang F, Huss M, Vega VB, Wong E, Orlov YL, Zhang W, Jiang J, Loh YH, Yeo HC, Yeo ZX, Narang V, Govindarajan KR, Leong B, Shahab A, Ruan Y, Bourque G, Sung WK, Clarke ND, Wei CL, Ng HH. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008 Jun 13;133(6):1106-17. doi: 10.1016/j.cell.2008.04.043. PMID:18555785 doi:http://dx.doi.org/10.1016/j.cell.2008.04.043
- ↑ 3.0 3.1 3.2 Zeineddine D, Hammoud AA, Mortada M, Boeuf H. The Oct4 protein: more than a magic stemness marker. Am J Stem Cells. 2014 Sep 5;3(2):74-82. eCollection 2014. PMID:25232507
- ↑ 4.0 4.1 Malakootian M, Mirzadeh Azad F, Naeli P, Pakzad M, Fouani Y, Taheri Bajgan E, Baharvand H, Mowla SJ. Novel spliced variants of OCT4, OCT4C and OCT4C1, with distinct expression patterns and functions in pluripotent and tumor cell lines. Eur J Cell Biol. 2017 Jun;96(4):347-355. doi: 10.1016/j.ejcb.2017.03.009. Epub, 2017 Apr 10. PMID:28476334 doi:http://dx.doi.org/10.1016/j.ejcb.2017.03.009
- ↑ Wang X, Dai J. Concise review: isoforms of OCT4 contribute to the confusing diversity in stem cell biology. Stem Cells. 2010 May;28(5):885-93. doi: 10.1002/stem.419. PMID:20333750 doi:http://dx.doi.org/10.1002/stem.419
- ↑ da Silva PBG, Teixeira Dos Santos MC, Rodini CO, Kaid C, Pereira MCL, Furukawa G, da Cruz DSG, Goldfeder MB, Rocha CRR, Rosenberg C, Okamoto OK. High OCT4A levels drive tumorigenicity and metastatic potential of medulloblastoma cells. Oncotarget. 2017 Mar 21;8(12):19192-19204. doi: 10.18632/oncotarget.15163. PMID:28186969 doi:http://dx.doi.org/10.18632/oncotarget.15163
- ↑ Villodre ES, Kipper FC, Pereira MB, Lenz G. Roles of OCT4 in tumorigenesis, cancer therapy resistance and prognosis. Cancer Treat Rev. 2016 Dec;51:1-9. doi: 10.1016/j.ctrv.2016.10.003. Epub 2016 Oct, 14. PMID:27788386 doi:http://dx.doi.org/10.1016/j.ctrv.2016.10.003
- ↑ Atlasi Y, Mowla SJ, Ziaee SA, Gokhale PJ, Andrews PW. OCT4 spliced variants are differentially expressed in human pluripotent and nonpluripotent cells. Stem Cells. 2008 Dec;26(12):3068-74. doi: 10.1634/stemcells.2008-0530. Epub 2008 , Sep 11. PMID:18787205 doi:http://dx.doi.org/10.1634/stemcells.2008-0530
- ↑ Hatefi N, Nouraee N, Parvin M, Ziaee SA, Mowla SJ. Evaluating the expression of oct4 as a prognostic tumor marker in bladder cancer. Iran J Basic Med Sci. 2012 Nov;15(6):1154-61. PMID:23653844
- ↑ Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002 Aug;3(2):167-70. doi: 10.1016/s1534-5807(02)00223-x. PMID:12194848 doi:http://dx.doi.org/10.1016/s1534-5807(02)00223-x
- ↑ Bowles J, Schepers G, Koopman P. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev Biol. 2000 Nov 15;227(2):239-55. doi: 10.1006/dbio.2000.9883. PMID:11071752 doi:http://dx.doi.org/10.1006/dbio.2000.9883
- ↑ Schaefer T, Lengerke C. SOX2 protein biochemistry in stemness, reprogramming, and cancer: the PI3K/AKT/SOX2 axis and beyond. Oncogene. 2020 Jan;39(2):278-292. doi: 10.1038/s41388-019-0997-x. Epub 2019 Sep, 2. PMID:31477842 doi:http://dx.doi.org/10.1038/s41388-019-0997-x
- ↑ Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 2008 Jan;26(1):101-6. doi: 10.1038/nbt1374. Epub 2007 Nov 30. PMID:18059259 doi:http://dx.doi.org/10.1038/nbt1374
- ↑ 14.0 14.1 14.2 14.3 14.4 14.5 14.6 Michael AK, Grand RS, Isbel L, Cavadini S, Kozicka Z, Kempf G, Bunker RD, Schenk AD, Graff-Meyer A, Pathare GR, Weiss J, Matsumoto S, Burger L, Schubeler D, Thoma NH. Mechanisms of OCT4-SOX2 motif readout on nucleosomes. Science. 2020 Apr 23. pii: science.abb0074. doi: 10.1126/science.abb0074. PMID:32327602 doi:http://dx.doi.org/10.1126/science.abb0074
- ↑ 15.0 15.1 15.2 15.3 15.4 Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015 Apr 23;161(3):555-568. doi: 10.1016/j.cell.2015.03.017. Epub 2015 Apr , 16. PMID:25892221 doi:http://dx.doi.org/10.1016/j.cell.2015.03.017
- ↑ 16.0 16.1 16.2 Mirny LA. Nucleosome-mediated cooperativity between transcription factors. Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22534-9. doi:, 10.1073/pnas.0913805107. Epub 2010 Dec 13. PMID:21149679 doi:http://dx.doi.org/10.1073/pnas.0913805107
- ↑ 17.0 17.1 17.2 Zeineddine D, Hammoud AA, Mortada M, Boeuf H. The Oct4 protein: more than a magic stemness marker. Am J Stem Cells. 2014 Sep 5;3(2):74-82. eCollection 2014. PMID:25232507
- ↑ 18.0 18.1 Zhang S, Cui W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells. 2014 Jul 26;6(3):305-11. doi: 10.4252/wjsc.v6.i3.305. PMID:25126380 doi:http://dx.doi.org/10.4252/wjsc.v6.i3.305
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs nameddois - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedtreze - ↑ Yamanaka S, Takahashi K. [Induction of pluripotent stem cells from mouse fibroblast cultures]. Tanpakushitsu Kakusan Koso. 2006 Dec;51(15):2346-51. PMID:17154061
- ↑ McCabe MJ, Alatzoglou KS, Dattani MT. Septo-optic dysplasia and other midline defects: the role of transcription factors: HESX1 and beyond. Best Pract Res Clin Endocrinol Metab. 2011 Feb;25(1):115-24. doi:, 10.1016/j.beem.2010.06.008. PMID:21396578 doi:http://dx.doi.org/10.1016/j.beem.2010.06.008